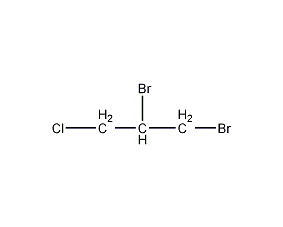
Structural formula
| Business number | 02AG |
|---|---|
| Molecular formula | C3H5Br2Cl |
| Molecular weight | 236.33 |
| label |
Dibromochloropropane, 3-Chloro-1,2-dibromopropane, Fumazone, Nemagon, Nemazont, Dibromochloropropane, soil fumigant, nematicides, Halogenated hydrocarbon solvents |
Numbering system
CAS number:96-12-8
MDL number:MFCD00039365
EINECS number:202-479-3
RTECS number:TX8750000
BRN number:1732077
PubChem number:24862844
Physical property data
1. Properties: light yellow liquid with pungent odor.
2. Relative density (g/mL, 20/4?): 2.081
3. Relative vapor density (g/mL, air=1): 8.2
4. Melting point (ºC): 6
5. Boiling point (ºC, normal pressure): 200
6. Boiling point (ºC, 1.33KPa): 196
7. Refractive index (25ºC): 1.553
8. Flash point (ºC): 77
9. Saturated vapor pressure (kPa, 21ºC): 0.12
p>
10. Solubility: Slightly soluble in water, soluble in ethanol, acetone, hydrocarbons, etc. Miscible with oils, dichloropropane and isopropyl alcohol.
Toxicological data
1. Irritation: Rabbit transdermal: 10 g severe irritation. Rabbit eye: 1% mild irritation.
2. Acute toxicity: Oral LD5O in rats: 170mg/kg
Oral LD5O in mice: 260mg/kg
Inhalation LC50 in rats: 154ppm, 4 hours 3. Harmful to human body. It is highly toxic and has been found to be carcinogenic in oral tests on mice.
Ecological data
This substance is harmful to the environment. Special attention should be paid to the pollution of water and air. It is extremely destructive to the atmospheric ozone layer.
Molecular structure data
1. Molar refractive index: 36.21
2. Molar volume (cm3/mol): 116.1
3. Isotonic specific volume (90.2K ): 291.6
4. Surface tension (dyne/cm): 39.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.35
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
p>
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 32
10. Number of isotope atoms: 0
11. Determine the atomic configuration Number of centers: 0
12. Uncertain number of atomic stereocenters: 1
13. Determined number of chemical bond stereocenters: 0
14. Uncertain Number of stereocenters of chemical bonds: 0
15, number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Add 3-chloropropene and bromine at normal pressure and 10-25°C, and the reaction product is purified by vacuum distillation. Add the original drug to the emulsifier and xylene, and emulsify at 55-60°C for half an hour to make an 80% emulsion. Raw material consumption quota: 3-chloropropene (92%) 310kg/t, liquid bromine 600kg/t
Purpose
Mainly used as solvent, soil fumigant, and nematicide.
extended-reading:https://www.newtopchem.com/archives/category/products/page/163extended-reading:https://www.newtopchem.com/archives/44507extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/NEWTOP8.jpgextended-reading:https://www.bdmaee.net/pc-cat-tka-metal-carboxylate-catalyst-nitro/extended-reading:https://www.bdmaee.net/low-density-sponge-catalyst-smp/extended-reading:https://www.bdmaee.net/pc-cat-np10-catalyst-n-dimethylaminopropyldiisopropanolamine/extended-reading:https://www.bdmaee.net/lupragen-n400-catalyst-trimethylhydroxyethyl-ethylene-diamine-basf/extended-reading:https://www.cyclohexylamine.net/strong-gel-amine-catalyst-bx405-low-odor-amine-catalyst-bx405/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/124-2.jpgextended-reading:https://www.newtopchem.com/archives/42953

