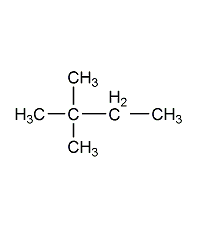
Structural formula
| Business number | 01K7 |
|---|---|
| Molecular formula | C6H14 |
| Molecular weight | 86.18 |
| label |
new hexane, Neohexane, gasoline additives |
Numbering system
CAS number:75-83-2
MDL number:MFCD00009321
EINECS number:200-906-8
RTECS number:EJ9300000
BRN number:1730736
PubChem number:24864699
Physical property data
1. Properties: colorless liquid with a slight odor at room temperature. [1]
2. Melting point (?): -99.9[2]
3. Boiling point (?): 49.7[3]
4. Relative density (water = 1): 0.649[4]
5. Relative vapor Density (air=1): 3.0[5]
6. Saturated vapor pressure (kPa): 36.9 (20?)[6]
7. Heat of combustion (kJ/mol): -4159.5[7]
8. Critical temperature (?): 216.2[8]
9. Critical pressure (MPa): 3.1[9]
10. Octanol/water partition coefficient: 3.82 [10]
11. Flash point (?): -47.8 (CC) [11]
12. Ignition temperature ( ?): 405[12]
13. Explosion upper limit (%): 7.0[13]
14. Explosion Lower limit (%): 1.2[14]
15. Solubility: insoluble in water, soluble in ethanol, ether, acetone, benzene, easily soluble in petroleum ether, tetrachlorine carbon. [15]
16. Flash point (ºC): 425
17. Critical density (g·cm-3) : 0.241
18. Critical volume (cm3·mol-1): 358
19. Critical compression factor: 0.279
20. Eccentricity factor: 0.234
21. van der Waals area (cm2·mol-1): 9.830×109
22. van der Waals volume (cm3·mol-1): 68.240 p>
23. Heat of evaporation (b.p,) (kJ/mol): 26.322
24. Heat of fusion (kJ/mol): 0.5798
25. Heat of generation ( 25 ºC, liquid, constant pressure) / (kJ·mol): -213.53
26. Specific heat capacity (25 ºC, liquid, constant pressure) / [kJ/(kg·K)]: 2.20
p>
27. Total combustion calorific value (KJ/mol): 4121.29
28. Minimum combustion calorific value (KJ/mol): 3842.97
29. Aniline point ( ºC): 81.2
30. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4175.76
31. Gas phase standard claimed heat (Enthalpy) (kJ·mol-1): -186.10
32. Gas phase standard entropy (J·mol-1·K -1): 358.4
33. Gas phase standard free energy of formation (kJ·mol-1): -9.9
34. Gas phase Standard hot melt (J·mol-1·K-1): 141.5
35. Liquid phase standard combustion heat (enthalpy) (kJ· mol-1): -4148.06
36. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -213.80
37. Liquid phase standard entropy (J·mol-1·K-1): 274.26
38. Liquid phase standard Free energy of formation (kJ·mol-1): -12.80
39. Liquid phase standard hot melt (J·mol-1·K-1)?189.67
Toxicological data
1. Acute poison?? No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No data yet
4. Other harmful effects[16] This substance may be harmful to the environment and should be Pay special attention to contamination of surface water, soil, atmosphere and drinking water.
Molecular structure data
1. Molar refractive index: 29.81
2. Molar volume (cm3/mol): 127.6
3. Isotonic specific volume (90.2K ): 265.9
4. Surface tension (dyne/cm): 18.8
5. Dielectric constant (F/m): 1.88
6. Polar Chemical rate (10-24cm3): 11.81
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 29.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The chemical properties are relatively stable, and halogenation reaction occurs under the action of sunlight or ultraviolet light to generate halogen derivatives. During the nitrification reaction, nitro compounds are produced. Solubility: Insoluble in water, miscible with alcohol, ether, acetone, benzene and petroleum ether. The solubility is similar to that of hexane and 2-methylpentane. Steam and air can easily form explosive mixtures, which can cause combustion and explosion when exposed to open flames or high heat.
2. Stability[17] Stability
3. Incompatible substances[18] Strong oxidants, strong acids, strong bases, halogens
4. Polymerization hazards[19] No polymerization
Storage method
Storage Precautions[20] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29?. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Refining method: Unsaturated compounds are removed by washing with concentrated sulfuric acid. Moisture can be removed with calcium chloride, phosphorus pentoxide, metallic sodium or solid desiccant.
Purpose
1. Gas chromatography analysis standards. Agrichemical intermediates. It has a high octane number and can be used as an additive for motor gasoline and aviation gasoline.
2. Used as solvent, additive for aviation gasoline and motor gasoline, also used in organic synthesis and used as gas chromatography comparison sample. [21]
extended-reading:https://www.newtopchem.com/archives/1604extended-reading:https://www.newtopchem.com/archives/44112extended-reading:https://www.bdmaee.net/toyocat-te-tertiary-amine-catalyst-tosoh/extended-reading:https://www.bdmaee.net/nt-cat-la-101-catalyst-cas31506-44-2-newtopchem/extended-reading:https://www.bdmaee.net/teda-l25b-polyurethane-tertiary-amine-catalyst-tosoh/extended-reading:https://www.bdmaee.net/cas-616-47-7/extended-reading:https://www.bdmaee.net/fentacat-f14-catalyst-cas112945-86-2-solvay/extended-reading:https://www.newtopchem.com/archives/212extended-reading:https://www.newtopchem.com/archives/1691extended-reading:https://www.newtopchem.com/archives/40409

