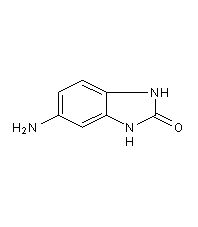
Structural formula
| Physical competition number | 028T |
|---|---|
| Molecular formula | C7H7N3O |
| Molecular weight | 149.15 |
| label |
5-amino-1,3-dihydro-2H-benzimidazol-2-one, 5-Aminobenzimidazolones, 5-amino-1,3-dihydro-2H-Benzimidazol-2-one |
Numbering system
CAS number:95-23-8
MDL number:MFCD00053555
EINECS number:202-401-8
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Character: light yellow or white crystal.
2. Density (g/mL, 20?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 239-244
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 40.26
2. Molar volume (cm3/mol): 109.3
3. Isotonic specific volume (90.2K ): 302.0
4. Surface tension (dyne/cm): 58.1
5. Polarizability (10-24cm3): 15.96
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 67.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 183
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
Benzimidazolone is produced by solid-phase reaction of o-phenylenediamine and urea at 150-250°C to deaminate the hydrogen ring, and then undergoes nitration and reduction to obtain it.
Purpose
Mainly used to prepare yellow to orange organic pigments.
extended-reading:https://www.newtopchem.com/archives/44319extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/129-1.jpgextended-reading:https://www.bdmaee.net/niax-bdma-liquid-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-BL-13-Niax-catalyst-A-133-Niax-A-133.pdfextended-reading:https://www.morpholine.org/amine-catalyst-dabco-8154-catalyst-dabco-8154/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-RP208-high-efficiency-reaction-type-equilibrium-catalyst-reaction-type-equilibrium-catalyst.pdfextended-reading:https://www.morpholine.org/3-morpholinopropylamine/extended-reading:https://www.bdmaee.net/niax-a-305-gel-catalyst-momentive/extended-reading:https://www.cyclohexylamine.net/lupragen-n105-pc-cat-nmm-dabco-nmm/extended-reading:https://www.newtopchem.com/archives/44293

