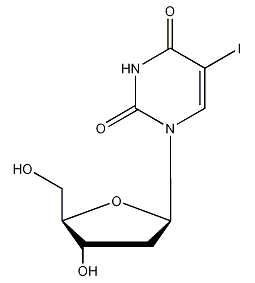
Structural formula
| Business number | 016D |
|---|---|
| Molecular formula | C9H11IN2O5 |
| Molecular weight | 354.1 |
| label |
2-deoxy-5′-iodouridine, 5′-iodo-2-deoxyuridine, 5′-iododeoxyuracil nucleoside, Herpes net; iodine deoxyuridine, 1-(2-Deoxy-?-D-ribofuranosyl)-5-iodouracil, 2′-Deoxy-5-iodouridine, antiviral drugs |
Numbering system
CAS number:54-42-2
MDL number:MFCD00134656
EINECS number:200-207-8
RTECS number:YU7700000
BRN number:30397
PubChem number:24896108
Physical property data
1. Properties: White crystalline powder.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 176~184? (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, normal pressure) 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation ( º): [?]D25+7.4° (C=0.108, in water), [?]D20 sup>+29.0° (C=1, in 1mol/L sodium carbonate)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure ( kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient relationship Value: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Solubility at 25°C (mg/ml): water 2, ethanol 2.6, ether 0.014, chloroform 0.03, acetone 1.6, ethyl acetate 1.8, dioxane 5.7. It is easily soluble in dilute sodium hydroxide solution and slightly soluble in dilute hydrochloric acid. The aqueous solution is more stable when it is acidic than when it is alkaline.
Toxicological data
None
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 64.75
2. Molar volume (cm3/mol): 164.8
3. Isotonic specific volume (90.2K): 504.2
4. Surface tension (dyne/cm): 87.4
5. Polarizability (10– 24cm3): 25.66
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -1
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecular polar surface area (TPSA): 99.1
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 386
10. Isotopic atoms Quantity: 0
11. Determine the number of atomic stereocenters: 3
12. Uncertain number of atomic stereocenters: 0
13. Determine chemical bond positions Number of stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a dry place at 4°C away from light.
Synthesis method
1. There are many production methods. Starting from propargyl alcohol, through oxidation; esterification, and then cyclization with 2- aminooxazoline nucleoside, can be obtained 2,2-oxo-pyrimidine nucleoside is then acylated, brominated, catalytically hydrogenated, and hydrolyzed to obtain iodine glycoside. 5′- deoxycytosine nucleotides can be prepared from fish essence, and iodine glycosides can also be prepared after further processing.
2.
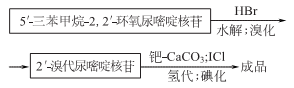
Purpose
1. Used for biochemical research. Tracheitis virus testing. Treat herpes simplex keratitis.
extended-reading:https://www.newtopchem.com/archives/1116
extended-reading:https://www.morpholine.org/2-dimethylamineethanol/
extended-reading:https://www.newtopchem.com/archives/39784
extended-reading:https://www.cyclohexylamine.net/reaction-type-catalyst-9727-polyurethane-amine-catalyst-9727/
extended-reading:https://www.bdmaee.net/fentacat-f50-catalyst-cas122695-73-9-solvay/
extended-reading:https://www.newtopchem.com/archives/44166
extended-reading:https://www.newtopchem.com/archives/687
extended-reading:https://www.bdmaee.net/fentacat-f33-catalyst-cas109526-41-1-solvay/
extended-reading:https://www.newtopchem.com/archives/516
extended-reading:https://www.newtopchem.com/archives/40454

