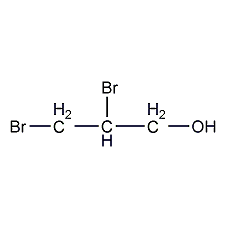
Structural formula
| Business number | 02AH |
|---|---|
| Molecular formula | C3H6Br2O |
| Molecular weight | 217.89 |
| label |
2,3-Dibromopropanol, Allyl alcohol dibromide, 2,3-Dibromopropanol, 2,3-Dibromo-1-propanol, 1,2-Dibromopropan-3-ol, flame retardant, Multifunctional solvent |
Numbering system
CAS number:96-13-9
MDL number:MFCD00004699
EINECS number:202-480-9
RTECS number:UB0175000
BRN number:1719127
PubChem number:24893858
Physical property data
1. Properties: colorless oily liquid.
2. Density (g/mL, 20/4?): 2.14
3. Boiling point (ºC, normal pressure): 219d
4. Boiling point (ºC, 1.6KPa): 101
5. Refractive index (n20ºC): 1.5625
6. Flash point (ºC): 110
4. p>
7. Solubility: Soluble in alcohol, ether, benzene, acetone and acetic acid, slightly soluble in water.
Toxicological data
1. Skin/eye irritation: Standard Draize test: rabbit, eye contact: 100?L/24H, severity of reaction: severe.
2. Acute toxicity: Oral LD50 in rats: 681mg/kg; Inhalation LC50 in rats: 9920mg/m3/4H; Intraperitoneal LD50 in mice: 125mg/ kg; Rabbit skin contact LD50: 316mg/kg;
3. Other multiple dose toxicity: Rat by inhalation TCLo: 500mg/kg/4H-3W-I; Rat skin contact TDLo: 11505mg /kg/13W-I;
4. Chronic toxicity/carcinogenicity: Rat skin contact TDLo: 47940mg/kg/51W-I; Mouse skin contact TDLo: 37170mg/kg/42W- I;
5. Mutagenicity: Microbial Salmonella Typhimurium mutation: 203?g/plate;
Microbial Salmonella Typhimurium mutation: 3300?g/plate; Hamster cell mutation experiment: 20?mol/L ;
E. coli mutation: 313?g/plate; Drosophila oral sex chromosome loss and non-disjunction experiment: 500ppm;
Drosophila oral genetic translocation experiment: 500ppm ;DNA damage to rat cells: 1?mol/L;
nbsp; DNA synthesis in rat liver: 100 ?mol/L; morphological transformation of hamster embryos: 500 ?mol/L;
6. Toxic when taken orally. Irritating to eyes, respiratory system and skin. There is the possibility of irreversible damage to the body.
Ecological data
None
Molecular structure data
1. Molar refractive index: 32.90
2. Molar volume (cm3/mol): 102.3
3. Isotonic specific volume (90.2K ): 269.8
4. Surface tension (dyne/cm): 48.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.04
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 32
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Stored in a cool and dark place.
Synthesis method
1. Obtained from the addition of propylene alcohol and bromine. Add propylene alcohol to the carbon tetrachloride solvent, perform bromination when adding bromine at 25-27°C, keep the temperature for 1 hour after adding (at 50±2°C), then distill to recover carbon tetrachloride, wash with water and sodium carbonate to pH The value is 7-8, dry, filter and clear, distill under reduced pressure, and collect the 140-160°C (2.66kPa) fraction as the finished product. It can also be produced without solvent: add propylene alcohol into the reaction pot, stir and cool. Add bromine dropwise below 10°C. The dropping speed is preferably such that the reaction temperature does not exceed 20°C. After the addition is completed, stir at room temperature for 1.5 hours, add water and stir for 10 minutes, let it stand, separate the oil layer, add sodium carbonate solution to neutralize it to neutrality. Leave to stand, separate the oil layer, distill under reduced pressure, and collect the 115-125°C (3.99-5.32kPa) fraction to obtain 2,3-dibromo-1-propanol.
2. Preparation method:
p>
In a reaction bottle equipped with a stirrer, thermometer and dropping funnel, add 58g (1.0mol) of propylene alcohol (2) and 100mL of carbon tetrachloride. While cooling in a water bath, slowly add 160g of bromine dropwise. (1.0 mol), control the dropping speed to keep the temperature of the reaction solution at 25~27°C. After the addition is completed, slowly increase the temperature to 50°C and keep the reaction for 1 hour. Carbon tetrachloride was evaporated under reduced pressure, and the residue was washed with water and sodium carbonate solution until neutral. Dry with anhydrous sodium sulfate and distill under reduced pressure. Collect the fraction at 115~125?/4~5.2kPa to obtain 175g of 2,3-1-propanol (1) with a yield of 80%. [1]
Purpose
Used in organic synthesis as solvent and flame retardant. Used as an intermediate for antidote dimercaprol and polyurethane foam, unsaturated polyester, phenolic, epoxy resin and polypropylene flame retardant products.
extended-reading:https://www.bdmaee.net/cas-1067-33-0/extended-reading:https://www.bdmaee.net/nt-cat-t26-catalyst-cas11207-74-9-newtopchem/extended-reading:https://www.bdmaee.net/pmdeta/extended-reading:https://www.bdmaee.net/high-rebound-delayed-catalyst-c-225/extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/05/Niax-catalyst-A-99.pdfextended-reading:https://www.newtopchem.com/archives/40300extended-reading:http://fh21com.cn”>extended-reading:https://www.newtopchem.com/archives/1114extended-reading:https://www.morpholine.org/127-08-2-2/extended-reading:https://www.bdmaee.net/dabco-xd-103-dabco-tertiary-amine-catalyst-catalyst-xd-103/

