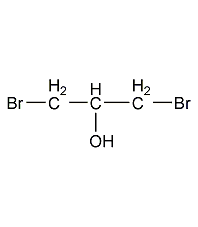
Structural formula
| Business number | 02AQ |
|---|---|
| Molecular formula | C3H6Br2O |
| Molecular weight | 217.89 |
| label |
1,3-dibromoisopropanol, 1,3-Dibromo-2-hydroxypropane, 1,3-Dibromo-2-hydroxypropane, 1,3-Dibromo-isopropylalcohol, Multifunctional solvents, Raw materials for organic synthesis |
Numbering system
CAS number:96-21-9
MDL number:MFCD00000216
EINECS number:202-489-8
RTECS number:UB0200000
BRN number:None
PubChem number:24863033
Physical property data
1. Properties: colorless oily liquid with special odor.
2. Density (g/mL, 25/4?): 2.1202
3. Boiling point (ºC, normal pressure): 219 (partially decomposed)
4. Boiling point (ºC, 5kPa): 124
5. Boiling point (ºC, 0.9kPa): 82~83
6. Refractive index (25ºC): 1.5495
7. Flash point (ºC): 46
8. Solubility: soluble in alcohol and ether, insoluble in water.
9. Relative density (20?, 4?): 2.1364
10. Refractive index at room temperature (n20): 1.5531
Toxicological data
1. Acute toxicity: Mouse intraperitoneal LD50: 150mg/kg;
2. Mutagenicity: Microbial Salmonella typhimurium mutation: 100?mol/plate; Cellular DNA inhibition: 1600?mol/L;
Ecological data
None
Molecular structure data
1. Molar refractive index: 32.90
2. Molar volume (cm3/mol): 102.3
3. Isotonic specific volume (90.2K ): 269.8
4. Surface tension (dyne/cm): 48.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.04
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 28
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocentersNumber of ? bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Irritating. It will oxidize and turn yellow if left in the air for a long time.
Storage method
None
Synthesis method
Obtained from the reaction of glycerol with red phosphorus and bromine. Mix glycerol and red phosphorus evenly, add bromine dropwise while stirring, and cause an exothermic reaction with a temperature of about 80°C. After adding the bromine, leave it overnight and distill under reduced pressure. The distillate is washed twice with 10% sea wave, dried with anhydrous sodium carbonate, filtered off the desiccant and then fractionated. The 110-112°C (2.66kPa) fraction is collected. Finished product.
Purpose
Used as solvent and organic synthesis intermediate.
extended-reading:https://www.bdmaee.net/polycat-15-catalyst-cas68479-98-1-evonik-germany/extended-reading:https://www.newtopchem.com/archives/859extended-reading:https://www.morpholine.org/polyurethane-catalyst-dabco-dc2-strong-gel-catalyst-dabco-dc2/extended-reading:https://www.bdmaee.net/2-ethylhexanoic-acid-potassium-cas-3164-85-0-dabco-k-15/extended-reading:https://www.newtopchem.com/archives/1896extended-reading:https://www.morpholine.org/catalyst-pc-41/extended-reading:https://www.newtopchem.com/archives/39159extended-reading:https://www.bdmaee.net/tetramethyl-13-diaminopropane/extended-reading:https://www.newtopchem.com/archives/39593extended-reading:https://www.newtopchem.com/archives/1081

