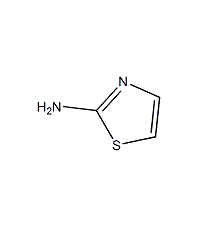
Structural formula
| Business number | 02B9 |
|---|---|
| Molecular formula | C3H4N2S |
| Molecular weight | 100.14 |
| label |
2-Aminothiazol |
Numbering system
CAS number:96-50-4
MDL number:MFCD00005325
EINECS number:202-511-6
RTECS number:XJ2100000
BRN number:105738
PubChem number:24846344
Physical property data
1. Properties: White to yellow crystals, gradually turning dark brown when exposed to air, easily sublimating.
2. Density (g/mL, 20?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 90
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 1.46kPa): 140
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg,ºC): Not determined
12. Saturated vapor pressure (kPa , ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in cold water, ethanol, easily soluble in hot water, Dilute inorganic acid.
Toxicological data
1. Acute toxicity: rat oral LD50: 480mg/kg; rat intravenous LD50: 570mg/kg; mouse peritoneal cavity LD50: 200mg/kg; cat oral LD50: 120mg/kg; rabbit oral LD50: 370mg /kg; guinea pig oral LDL0: 120mg/kg; 2. Other multiple dose toxicity: rabbit oral TDLo: 4500 mg/kg/56D-I; rabbit inhalation TCLo: 200 mg/m3/7H/61D-I; guinea pig inhalation TCLo: 200 mg/m3/7H/30D-I; TCLo inhaled by guinea pigs: 25mg/m3/7H/62D-I; 3. Mutagenicity: Mutant microorganism test: Bacteria – Salmonella typhimurium, 3333?g/plate; Mutant microorganism test : Klebsiella pneumoniae, 1mmol/L; mutation test of microorganisms: mouse lymphocytes, 1214mg/L; mutation test in mammalian body: mouse lymphocytes, 557mg/L;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 26.96
2. Molar volume (cm3/mol): 74.4
3. Isotonic specific volume (90.2K ): 210.4
4. Surface tension (dyne/cm): 63.9
5. Polarizability (10-24cm3): 10.68
Compute chemical data
1.?Reference value for water parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 67.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 48.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid light. Avoid contact with strong oxidants, strong acids, acid chlorides, and acid anhydrides.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained by the cyclization of thiourea and chloroacetaldehyde (or ethanol and chlorine, or ?, ? dichloroethyl ether). Add hot water, thiourea and ?,?-dichloroethyl ether to the reactor. Reflux with stirring for 2h. Cool and add sodium hydroxide solution through the dropping funnel to make the solution alkaline and precipitate 2-aminothiazole crystals. Then add diethyl ether to dissolve. The ether layer was separated, washed with water, dried over anhydrous sodium sulfate, and the ether was evaporated to obtain crude product. Recrystallize with ethanol to obtain yellow crystals. The yield is 80% and the melting point is 90°C.
Purpose
Used to synthesize sulfathiazole and antithyroid drugs, and as an intermediate in organic synthesis.
extended-reading:https://www.bdmaee.net/dibutyloxostannane/extended-reading:https://www.newtopchem.com/archives/category/products/page/120extended-reading:https://www.newtopchem.com/archives/44293extended-reading:https://www.cyclohexylamine.net/cas-103-83-3-bdma-benzyldimethylamine/extended-reading:https://www.newtopchem.com/archives/915extended-reading:https://www.newtopchem.com/archives/45062extended-reading:https://www.newtopchem.com/archives/44723extended-reading:https://www.bdmaee.net/polyurethane-monosodium-glutamate/extended-reading:https://www.newtopchem.com/archives/40394extended-reading:https://www.newtopchem.com/archives/177

