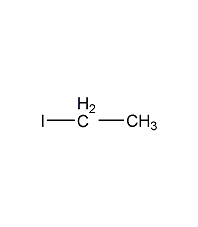
Structural formula
| Business number | 01HU |
|---|---|
| Molecular formula | C2H5I |
| Molecular weight | 155.97 |
| label |
ethyl iodide, Ethyl iodide, Ethyl iodide, Hydrodic ether, 1-Iodoethane, Monoiodoethane, Ethyl iodide, Aliphatic halogenated derivatives |
Numbering system
CAS number:75-03-6
MDL number:MFCD00001091
EINECS number:200-833-1
RTECS number:KI4750000
BRN number:505934
PubChem number:24845689
Physical property data
1. Characteristics: Colorless, clear and heavy liquid with an ether smell. [1]
2. Melting point (?): -108[2]
3. Boiling point (?): 69~73[3]
4. Relative density (water=1): 1.95[4]
5. Relative vapor density (air = 1): 5.38[5]
6. Saturated vapor pressure (kPa): 13.33 (18.0?)[6]
7. Heat of combustion (kJ/mol): -1490.6[7]
8. Critical pressure (MPa): 5.99[ 8]
9. Octanol/water partition coefficient: 2.0[9]
10. Flash point (?): >71 [10]
11. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, and hydrocarbons. [11]
12. Relative density (20?, 4?): 1.9357
13. Refractive index at room temperature (n20
sup>): 1.5133
14. Refractive index at room temperature (n25): 1.5101
15. Liquid phase standard claims heat (enthalpy) (kJ· mol-1): -39.5
16. Solubility parameter (J·cm-3)0.5: 19.078
17. van der Waals area (cm2·mol-1): 5.950×109
18. van der Waals volume (cm3·mol-1): 43.080
19. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -7.5
20. Gas phase standard entropy (J·mol-1·K-1): 295.63
21. Gas phase standard formation free energy (kJ·mol-1): 20.7
22. Gas phase standard hot melt ( J·mol-1·K-1)?65.33
Toxicological data
1. Acute toxicity[12]
LD50: 330mg/kg (oral in rats); 560mg/kg (oral in mice) Oral)
LC50: 65000mg/m3 (rat inhalation, 1/2h)
2. Irritation Temporary No information
3. Mutagenicity [13] Mutagenicity of Escherichia coli: 20 ?mol/L; DNA damage: 1 ?mol/L.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[14] This substance hasThe environment is hazardous and attention should be paid to atmospheric pollution.
Molecular structure data
1. Molar refractive index: 24.28
2. Molar volume (cm3/mol): 80.3
3. Isotonic specific volume (90.2K ): 185.7
4. Surface tension (dyne/cm): 28.5
5. Polarizability (10-24cm3): 9.62
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[15] Stable
2. Incompatible substances[16] Strong oxidizing agent, strong alkali
3. Conditions to avoid contact [17] Heat, light, contact with air
4. Polymerization hazard[18] No polymerization
5. Decomposition products[19] Hydrogen iodide
Storage method
1. Packed in 25~500ml glass bottles and protected by wooden boxes or cartons. Store in a cool, dry and ventilated warehouse, away from fire, heat sources and protected from light. Do not mix with edible chemical raw materials.
2. Storage precautions [20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Avoid light. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of ethanol and phosphorus triiodide. Put industrial ethanol and red phosphorus into the reaction bottle, heat to reflux on a water bath, and slowly add iodine granules. After the addition is completed, continue refluxing for 3 hours. The reaction solution is then distilled to evaporate the yellow crude product, which is washed and dried to obtain the finished product. In addition, ethyl iodide can also be produced by the addition of ethylene and hydrogen iodide. The reaction formula is:
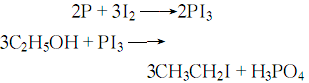
2. It is produced by the addition reaction of ethylene and hydriodic acid.

3. From ethanol and triiodine Derived from the reaction of phosphorus. The operation process is as follows: place ethanol and red phosphorus in a reaction bottle, heat and reflux on a water bath, slowly add iodine particles, and continue to reflux. Then the reaction liquid is evaporated to obtain a yellow crude product, which is then washed, dried and filtered to obtain the finished product.
Purpose
1. Used as chemical reagents and pharmaceutical penetration aids (to measure cardiac blood output). Also used in organic synthesis and as reagents. Goiter treatment drugs, plant growth stimulating hormone, etc.
2. Determine the refractive index of minerals. Calibrate the refractometer. Separate minerals. Measurement of cardiac blood output.
3. Used as analytical reagents, such as for measuring refractive index.
4. Used in medicine and organic synthesis. [21]
extended-reading:https://www.newtopchem.com/archives/category/products/page/4extended-reading:https://www.newtopchem.com/archives/category/products/page/6extended-reading:https://www.cyclohexylamine.net/category/product/page/21/extended-reading:https://www.morpholine.org/elastomer-environmental-protection-catalyst-nt-cat-e-129/extended-reading:https://www.cyclohexylamine.net/potassium-acetate-glycol-solution-polycat-46/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-NE500-non-emission-amine-catalyst-NE500-strong-gel-amine-catalyst-NE500.pdfextended-reading:https://www.newtopchem.com/archives/category/products/page/99extended-reading:https://www.bdmaee.net/dabco-tl-low-odor-tertiary-amine-catalyst-dabco-low-odor-tertiary-amine-catalyst/extended-reading:https://www.bdmaee.net/catalyst-a300-a300-nt-cat-300/extended-reading:https://www.bdmaee.net/dimethylbis1-oxoneodecyloxystannane/

