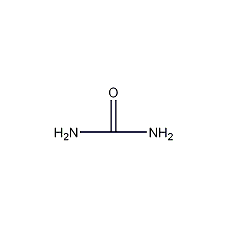
Structural formula
| Business number | 018E |
|---|---|
| Molecular formula | CH4N2O |
| Molecular weight | 60.06 |
| label |
carbonamide, urea, carbamide urea, Urea (industrial use), Urea, carbonamide, carbonamide, Aminomethamine, Alpharate, Carbonyldiamide, Carbamide, Ureaphil |
Numbering system
CAS number:57-13-6
MDL number:MFCD00008022
EINECS number:200-315-5
RTECS number:YR6250000
BRN number:635724
PubChem number:24900617
Physical property data
1. Character: White, tasteless, odorless crystal or powder
2. Density (g/mL, 25 /4?): 1.323
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 132.9?. When heated above the melting point, it decomposes into biuret, chlorine and cyanuric acid.
5. Boiling point (ºC, normal pressure): 383
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.4299
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
p>
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, ethanol and benzene, 1g of this product is soluble in 1ml, 10ml95% ethanol, 1ml95% boiling water Ethanol, 20ml absolute ethanol, 6ml methanol and 2ml glycerin. Slightly soluble in ether, insoluble in chloroform.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Moore’s foldEmissivity: 13.78
2. Molar volume (cm3/mol): 49.5
3. Isotonic volume (90.2K): 135.0
4. Surface tension (dyne/cm): 55.3
5. Polarizability (10-24cm3): 5.46
Compute chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): -1.4
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 69.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 29
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has certain hygroscopicity and will decompose when heated above the melting point.
Storage method
1. This product should be sealed and stored in a cool, dry place.
Synthesis method
1. Urea is the final product of protein metabolism in mammals. In 1922, industrial production of urea using ammonia and carbon dioxide was realized in Germany. Ammonia reacts with carbon dioxide to form amine carbamate, which is then dehydrated to form urea.
2.Ammonia reacts with carbon dioxide to form ammonium carbamate, which is then dehydrated to form urea.
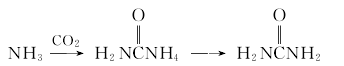 The purified ammonia and carbon dioxide are mixed and sent to the synthesis tower at a molar ratio of 2.8 to 4.5. The pressure in the tower is 13.8 to 24.6MPa, the temperature is 180 to 200°C, and the reaction material residence time is 25 to 40 minutes to obtain excess ammonia. and urea solution of ammonium carbamate (urea solution). After decompression and cooling, the urea liquid after separating ammonia and ammonium carbamate is evaporated and concentrated to more than 99.5%, and then granulated in a granulation tower to obtain the finished urea.
The purified ammonia and carbon dioxide are mixed and sent to the synthesis tower at a molar ratio of 2.8 to 4.5. The pressure in the tower is 13.8 to 24.6MPa, the temperature is 180 to 200°C, and the reaction material residence time is 25 to 40 minutes to obtain excess ammonia. and urea solution of ammonium carbamate (urea solution). After decompression and cooling, the urea liquid after separating ammonia and ammonium carbamate is evaporated and concentrated to more than 99.5%, and then granulated in a granulation tower to obtain the finished urea.
There are currently 4 popular processes in the world: the CO2 stripping process of Stamicarbon in the Netherlands, and Snam in Italy. Progeti’s NH3 stripping process, Japan’s Mitsui Toyo’s ACES process and Italy’s Monte Edison’s IDR process. Foreign countries have recently successfully developed a new process for high-efficiency combined urea production. This process is a major improvement based on the aqueous solution circulation method. Its scale can be large or small, and it is especially suitable for the technological transformation and production conversion of medium and small nitrogen fertilizer enterprises in my country. Its consumption indicators per ton of urea are almost indistinguishable from those of the above-mentioned advanced processes. The process flow is as follows: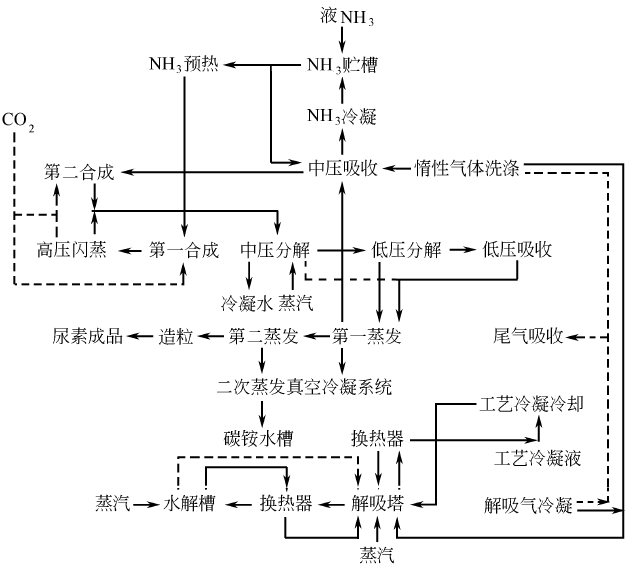
3 .Calcium cyanamide generates cyanamide under the action of sulfuric acid, and then reacts with water to form urea:
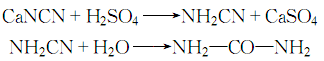
After decolorization and recrystallization, the finished product is obtained
Purpose
1. Urea is mainly used as chemical fertilizer. Industrially, it is also used as a raw material for the manufacture of urea-formaldehyde resin, polyurethane, and melamine-formaldehyde resin. It is also widely used in medicine, explosives, tanning, flotation agents, pigments, and dewaxing of petroleum products. When urea is heated to 200°C, it generates solid tripolychloric acid (cyanuric acid). Derivatives of cyanuric acid: trichloroisocyanuric acid, sodium dichloroisocyanurate, tri(2-hydroxyethyl) isocyanurate, tri(allyl)isocyanurate, tri(3,5-di Tert-butyl-4-hydroxybenzyl) isocyanate, triglycidyl isocyanurate, melamine cyanurate complex, etc. have many important applications. The first two are new high-end disinfectants and bleaches. The total production capacity of trichloroisocyanuric acid in the world exceeds 80,000 tons.
2.Test antimony and tin, and determine lead, copper, gallium, phosphorus, iodide, and nitrate.
3.As a non-protein nitrogen source for ruminants, it is added to feed.
4.Used as analytical reagents and stabilizers. Also used in organic synthesis.
5.Used as a solubilizer for liquid detergents. Used in the preparation of toothpaste, it can inhibit the growth of lactobacilli and dissolve the plaque on the tooth surface, thereby acting as an anti-acid and desensitizing agent. Also used in cosmetics. Also used as a reagent for testing antimony and tin. Used to prepare strontium salts, fireworks, etc.
6.Used for moisturizing and moisturizing in cosmetics.
7.It has a brightening effect on chemical polishing of steel and stainless steel, and is used as a corrosion inhibitor in metal pickling. Preparation of palladium activation solution.
extended-reading:https://www.bdmaee.net/dimethyl-tin-oxide-2273-45-2-cas2273-45-2-dimethyltin-oxide/extended-reading:https://www.bdmaee.net/fentacat-f50-catalyst-cas122695-73-9-solvay/extended-reading:https://www.newtopchem.com/archives/44704extended-reading:https://www.bdmaee.net/polyurethane-catalyst-smp-catalyst-smp-sponge-catalyst-smp/extended-reading:https://www.cyclohexylamine.net/category/product/page/22/extended-reading:https://www.bdmaee.net/tertiary-amine-composite-catalyst/extended-reading:https://www.bdmaee.net/niax-c-5-intense-foaming-catalyst-pentamethyldiethylenetriamine-momentive/extended-reading:https://www.cyclohexylamine.net/dabco-mp602-delayed-amine-catalyst/extended-reading:https://www.bdmaee.net/dmdee/extended-reading:https://www.cyclohexylamine.net/high-quality-cas-110-95-2-tetramethyl-13-diaminopropane-tmeda/

