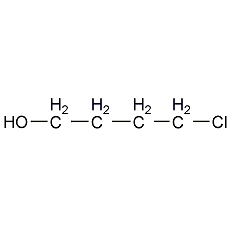
Structural formula
| Business number | 018G |
|---|---|
| Molecular formula | C4H9ClO |
| Molecular weight | 108.57 |
| label |
Trichlorobutanol, Chlorohydrin;, 1,1,1-Trichloro-2-methyl-2-propanol, preservative |
Numbering system
CAS number:57-15-8
MDL number:None
EINECS number:200-317-6
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless crystal. It exists in two types of crystals: half-molecule crystal water type and anhydrous type.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): The melting point of the type containing half a molecule of crystal water is 78°C, and the melting point of the anhydrous type is 97
5. Boiling point (ºC, normal pressure): 167
6. Boiling point (ºC, 32.7kPa): 135
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC) : Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: The type containing half a molecule of crystal water is slightly soluble in water (1:250), easily soluble in ethanol (1:1), glycerol (1:10), ether, chloroform and volatile oils. The anhydrous type is easily soluble in hot water, 1g can be dissolved in 1ml ethanol or 10ml glycerol, and soluble in ether, petroleum ether, acetone, chloroform, glacial acetic acid, and oils.
Toxicological data
1. Skin or eye irritation: Rabbit, skin contact, standard Draize test, 850ug, mild reaction; Rabbit, eye contact, standard Draize test, 9180ug/30, mild reaction 2. Acute toxicity: dog oral LDLo : 238mg/kg; rabbit oral LDLo: 213mg/kg; frog parenteral LDLo: 800mg/kg 3. Mutagenicity: mutation microorganismsTEST system: bacteria – Salmonella typhimurium: 20umol/plate
Ecological data
Temporarily??
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 26.96
2. Molar volume (cm3/mol): 103.4
3. Isotonic specific volume (90.2K): 246.4
4. Surface tension (dyne/cm): 32.2
5. Polarizability (10-24cm3): 10.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 83.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable properties under normal temperature and pressure.
Storage method
Store in a cool and dry place.
Synthesis method
1. Obtained from the reaction of acetone and chloroform: Add chloroform and acetone into the reaction pot at an ingredient ratio of 1:0.5, cool to 8°C, slowly add potassium hydroxide while stirring, and control the temperature not to exceed 15°C. After the addition is completed, maintain stirring at the same temperature for 2 hours, discharge, filter, and concentrate the filtrate until no distillate drips out. Stop heating, cool down to 25°C, add ice water, stir, precipitate crystals, centrifuge crystallization, centrifuge separation, wash the filter cake with distilled water until there is no chloride ion. Dry at 60-65°C for about 2 hours, sieve and package. The yield is 56% (based on chloroform). Raw material consumption quota: chloroform 1247kg/t, acetone 1688kg/t, potassium hydroxide 285kg/t.
Purpose
1. Mainly used as pharmaceutical raw materials to make antiseptics, antiemetics, and local analgesics. Its 1% aqueous solution or 5%-10% ointment can be used for disinfection and sterilization. It can also be used in organic synthesis.
2.Used as antiseptics, anesthetics, and cosmetic preservatives. The maximum allowable content (mass fraction) of cosmetics is 0.5%.
extended-reading:https://www.newtopchem.com/archives/1902extended-reading:https://www.cyclohexylamine.net/delayed-equilibrium-catalyst-dabco-catalyst/extended-reading:https://www.bdmaee.net/dabco-ncm-pc-cat-ncm-polyester-sponge-catalyst-dabco-ncm/extended-reading:https://www.cyclohexylamine.net/efficient-reaction-type-equilibrium-catalyst-reactive-equilibrium-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/63.jpgextended-reading:https://www.newtopchem.com/archives/40546extended-reading:https://www.bdmaee.net/niax-a-310-balanced-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/kosmos-19-catalyst-cas121-73-6-degussa-ag/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/07/37.jpgextended-reading:https://www.bdmaee.net/niax-a-107-delayed-amine-catalyst-momentive/

