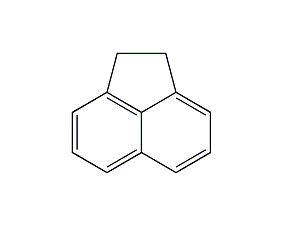
Structural formula
| Business number | 01TN |
|---|---|
| Molecular formula | C12H10 |
| Molecular weight | 154.21 |
| label |
naphthoethane, Naphthaethyl ring, Versaphos, naphthyl ring, rylene, acenaphthene, Killer, acenaphthene, Er, rylene, Ethane naphthalene, Naphthylene ethylene, 1,2-Dihydroacemaphthylene, peri-Ethylenenaphthalene, 1,8-Ethylenenaphthalene, Aromatic hydrocarbons |
Numbering system
CAS number:83-32-9
MDL number:MFCD00003807
EINECS number:201-469-6
RTECS number:AB1000000
BRN number:386081
PubChem ID:None
Physical property data
1.Characteristics: white needle-like crystals. [1]
2. Melting point (?): 95[2]
3. Boiling point (?): 279 [3]
4. Relative density (water = 1): 1.024[4]
5. Relative vapor density (Air=1): 5.32[5]
6. Saturated vapor pressure (kPa): 1.33 (131.2?)[6]
7. Critical pressure (MPa): 3.1[7]
8. Octanol/water partition coefficient: 3.92[8]
9. Flash point (?): 120[9]
10. Explosion limit (%): 5.3[10]
11. Lower explosion limit (%): 0.8[11]
12. Solubility: insoluble in water, slightly soluble in ethanol, Soluble in chloroform, benzene, toluene, glacial acetic acid and petroleum ether. [12]
13. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -6307.3
14. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 156.0
15. Crystal phase standard combustion heat (enthalpy) (kJ·mol -1): -6221.6
16. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): 70.3
Toxicological data
1. Acute toxicity[13] LD50: 600mg/kg (rat abdominal cavity)
2. Irritation strong> No data yet
3. Mutagenicity[14] Microbial mutagenicity: Salmonella typhimurium spp. 0.5nmol/dish (48h). Cytogenetic analysis: hamster lung 10mmol/L (6h)
Ecological data
1. Ecotoxicity[14]
LC50: 1.7mg/L (72h), 1.6mg/L (96h) (blackhead Minnow);
7.2mg/L (24h), 1.7mg/L (96h) (bluegill sunfish, static);
1.57mg/L (24h), 1.13mg/L??48h), 0.8mg/L (72h), 0.67mg/L (96h) (rainbow trout); 0.96mg/L (96h) (sugar shrimp, static)
EC50: 0.52mg/L (96h) (green algae); 0.5mg/L (96h) (Skeletonema costatum)
2. Biodegradability[15]
Aerobic biodegradation (h): 295~2448
Anaerobic biodegradation (h): 1180~9792
3. Abiotic degradation Properties[16]
Aqueous phase photolysis half-life (h): 3~60
Photolysis maximum light absorption wavelength range (nm ): 288~320
Photooxidation half-life in air (h): 0.879~8.79
Molecular structure data
1. Molar refractive index: 51.65
2. Molar volume (cm3/mol): 134.9
3. Isotonic specific volume (90.2K ): 357.2
4. Surface tension (dyne/cm): 49.2
5. Dielectric constant (F/m): 3.11
6. Polar Chemical rate (10-24cm3): 20.47
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 155
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Flammable and irritating to eyes, respiratory system and skin. Appropriate protective clothing should be worn for heavy use. Avoid contact with eyes and skin. In case of contact with eyes, rinse immediately with plenty of water.
2. Stability[17] Stable
3. Incompatible substances[18] Strong oxidizing agent
4. Conditions to avoid contact[19] Heat
5. Aggregation hazards[20] No aggregation
Storage method
Storage Precautions[21] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 35?. The packaging is sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. In high-temperature tar, it contains about 1.2%-1.8% acenaphthene. The wash oil separated from coal tar distillation is divided into various narrow fractions by distillation method, and industrial acenaphthene is produced from the 270-280°C fraction.
2. It can also be produced by the interaction between naphthalene and ethylene.
Purpose
1. Determination of aromatic aldehydes. Fungicides. Manufacturing of dyes and plastics.
2. Used as dye intermediates, pesticides, fungicides, etc. [22]
extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/2-3.jpgextended-reading:https://www.newtopchem.com/archives/40426extended-reading:https://www.bdmaee.net/tmr-4-dabco-tmr-4-trimer-catalyst-tmr-4/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/7-1.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/115-12.jpgextended-reading:https://www.newtopchem.com/archives/44261extended-reading:https://www.newtopchem.com/archives/44006extended-reading:https://www.newtopchem.com/archives/category/productsextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/102-7.jpgextended-reading:https://www.bdmaee.net/niax-a-1/

