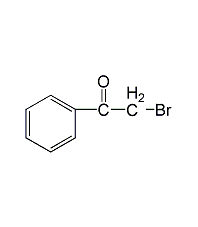
Structural formula
| Business number | 01G1 |
|---|---|
| Molecular formula | C5H7BrO3 |
| Molecular weight | 195.01 |
| label |
Ethyl bromopyruvate, BrCH2COCOOC2H5 |
Numbering system
CAS number:70-23-5
MDL number:MFCD00000204
EINECS number:200-729-6
RTECS number:None
BRN number:1760158
PubChem ID:None
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25/4?): 1.56
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 1.33kPa): 98? 100
7. Refractive index: 1.4690
8. Flash point (ºC): 98
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 34.74
2. Molar volume (cm3/mol): 124.8
3. Isotonic specific volume (90.2K ): 313.0
4. Surface tension (dyne/cm): 39.5
5. Polarizability (10-24cm3): 13.77
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 2
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 121
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed with nitrogen and protected from light at 0~4?.Save.
Synthesis method
1. Obtained from ethyl lactate through oxidation and bromination: ethyl lactate, NBS (N-bromododecimide) and tetrachloride Add carbon dioxide into a dry reaction pot and heat to reflux for 5.5 hours while stirring. Cool and filter, evaporate the carbon tetrachloride from the filtrate, distill under reduced pressure, and collect the 71~73°C (0.67kPa) fraction, which is ethyl bromopyruvate.
2. Preparation method:
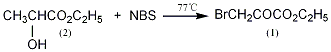
Into a reaction bottle equipped with a stirrer, thermometer, and reflux condenser, add 66g (0.56mol) of freshly steamed ethyl lactate (2), 900mL of carbon tetrachloride, and 200g of NBS ( 1.14mol), heated to (77±1)°C in a water bath, stirred and refluxed for 5 to 6 hours, until the reaction solution changed from red to orange. Hydrogen bromide gas is released during the reaction. Place in ice-salt bath overnight and filter out succinimide. After the filtrate is distilled under normal pressure to recover carbon tetrachloride, it is distilled under reduced pressure and the fraction at 71~73?/0.67kPa is collected to obtain a yellow transparent liquid ethyl bromopyruvate (1)?56.8g, The yield is 52%. nD251.465?1.4673. Note: ? It has a strong tear-inducing effect. Store it in a refrigerator after being airtight. [1]
Purpose
Organic Synthesis.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/stannous-octoate-CAS-301-10-0–T-9.pdfextended-reading:https://www.newtopchem.com/archives/208extended-reading:https://www.bdmaee.net/dimethyltin-dioctanoate/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/68.jpgextended-reading:https://www.bdmaee.net/dabco-ne200-catalyst-cas10317-48-7-evonik-germany/extended-reading:https://www.bdmaee.net/low-odor-reactive-catalyst/extended-reading:https://www.bdmaee.net/fascat4201-catalyst-cas-818-08-6-dibutyl-tin-oxide/extended-reading:https://www.newtopchem.com/archives/44729extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-2040-low-odor-amine-catalyst-low-odor-catalyst.pdfextended-reading:https://www.bdmaee.net/nt-cat-tmpda-catalyst-cas10294-43-5-newtopchem/