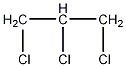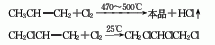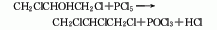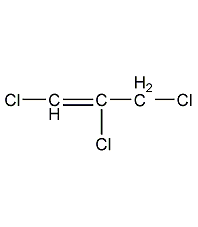
Structural formula
| Business number | 02AN |
|---|---|
| Molecular formula | C3H3Cl3 |
| Molecular weight | 145 |
| label |
Aliphatic halogenated derivatives |
Numbering system
CAS number:96-19-5
MDL number:None
EINECS number:None
RTECS number:UD2450000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 20?): 1.414
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 142
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: 1.5030
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water. Soluble in ethanol and chloroform.
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 10mg/24H, severity of reaction: severe.
Standard Draize test: Rabbit, eye contact: 50 mg, severity of reaction: moderate.
2. Acute toxicity: Rat oral LD50: 616mg/kg; Rat inhalation LCLo: 500ppm/4H; Rabbit skin contact LD50: 640?L/kg;
3 , Other multi-dose toxicity: rats inhaled TCLo: 36ppm/6H/4W-C;
4. Mutagenicity
Mutation of microorganism Salmonella typhimurium: 1?mol/plate;
DNA inhibition of human HeLa cells: 1700?mol/L;
Ecological data
None
Molecular structure data
1. Molar refractive index: 30.39
2. Molar volume (cm3/mol): 105.6
3. Isotonic specific volume (90.2K ): 250.2
4. Surface tension (dyne/cm): 31.4
5. Polarizability (10-24cm3): 12.04
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5, Number of tautomers:
6, Topological molecular polar surface area (TPSA): 0
7 , Number of heavy atoms: 6
8, Surface charge: 0
9, Complexity: 57.1
10, Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
p>
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Toxic, irritating to human lungs and stomach. Because the product contains a small amount of chloroacetone, it has tear-inducing properties. Protective equipment should be worn during operation and should be replaced immediately if it is attached to clothing.
?
Storage method
For personal use, it is transported by pipeline; for export, it can be packed in iron drums.
Synthesis method
The preparation method is to add tetrachloropropane and ethanol into the reaction kettle, stir and heat to reflux, add potassium hydroxide in batches within 1 hour, complete the addition and reflux for 2 hours, cool, filter, and wash the filtrate twice with water. The water layer is extracted with dichloroethane, combined with the oil layer, desolvated, and distilled under reduced pressure. The 74-91°C/13.3 kPa fraction is collected as the finished product.
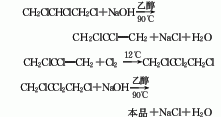
Purpose
Used as an intermediate for the herbicide oatmein.
It is mainly used as an intermediate for the pesticides and herbicides Ovenamidine and Odontamine No. 1, and is also a raw material for manufacturing special plastics.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyltin-dichloride-CAS683-18-1-di-n-butyltin-dichloride.pdfextended-reading:https://www.bdmaee.net/nt-cat-la-210-catalyst-cas10861-07-1-newtopchem/extended-reading:https://www.bdmaee.net/tris-dimethylaminopropyl-hexahydrotriazine-cas-15875-13-5-triazine-catalyst/extended-reading:https://www.cyclohexylamine.net/dabco-mp601-delayed-polyurethane-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/76extended-reading:https://www.newtopchem.com/archives/44564extended-reading:https://www.newtopchem.com/archives/44570extended-reading:https://www.newtopchem.com/archives/185extended-reading:https://www.bdmaee.net/dinbutyltindichloride/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-BL-17-Niax-A-107-Jeffcat-ZF-54.pdf