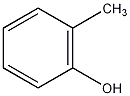
Structural formula
| Physical competition number |
0295 |
| Molecular formula |
C7H8O |
| Molecular weight |
108.14 |
| label |
o-cresol,
O-steamed lignooleic acid,
2-Cresol,
2-methylphenol,
2-Cresol,
2-Methylphenol,
o-Methylphenol,
o-Hydroxy-toluene,
Aromatic halogen derivatives
|
Numbering system
CAS number:95-48-7
MDL number:MFCD00002226
EINECS number:202-423-8
RTECS number:GO6300000
BRN number:506917
PubChem number:24867813
Physical property data
1. Characteristics: white crystal with aromatic odor. [1]
2. Melting point (?): 29.8~31[2]
3. Boiling point (?) ?191~192[3]
4. Relative density (water=1): 1.05[4]
5 .Relative vapor density (air=1): 3.72[5]
6. Saturated vapor pressure (kPa): 0.133 (38.2?)[6]
7. Heat of combustion (kJ/mol): -3689.8[7]
8. Critical temperature (?): 424.5 [8]
9. Critical pressure (MPa): 5.01[9]
10. Octanol/water partition coefficient: 1.95 [10]
11. Flash point (?): 81 (CC) [11]
12. Ignition Temperature (?): 598[12]
13. Explosion limit (%): 7.6[13]
14 .Lower explosion limit (%): 1.4 (148?)[14]
15. Solubility: Slightly soluble in water, soluble in ethanol, ether, chloroform, etc. [15]
16. Viscosity (mPa·s, 20ºC): 9.56
17. Heat of evaporation (KJ/mol, b.p.): 415.0
18. Specific heat capacity (KJ/(kg·K), constant pressure): 2.09
19. Electrical conductivity (S/m, 25ºC): 0.127×10-8
20. Solubility (%, 25ºC, water): 2.2
21. Refractive index at room temperature (n25): 1.5399 p>
22. Relative density (25?, 4?): 1.03535
23. Eccentricity factor: 0.434
24. Gas phase standard Heat of combustion (enthalpy) (kJ·mol-1): -3769.32
25. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -128.57
26. Gas phase standard entropy (J·mol-1·K-1): 352.70
27. Gas phase standard formation free energy (kJ·mol-1): -34.3
28. Gas phase standard hot melt (J·mol-1·K-1): 127.30
29. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -3693.30
30. Crystal phase standard claim heat (enthalpy) (kJ·mol-1): -204.60
31. Crystal phase standard entropy (J· mol-1·K-1): 165.44
32. Crystal phase standard formation free energy (kJ·mol-1): -55.69
33. Crystal phase standard hot melt (J·mol-1·K-1): 154.56
Toxicological data
1. Acute toxicity: Rat oral LD50: 121mg/kg; Mouse oral LC50: 344mg/kg; Rat inhalation LDL0: 940mg/kg; Rabbit transdermal LD50: 890mg/kg; Rat transdermal LD50: 620mg/kg; Rat inhalation LD50: >1220mg/kg m3/1H;
2. It has strong irritating and corrosive effects on skin and mucous membranes. The olfactory threshold concentration is 7.956×10-4mg/m3, and the maximum allowable concentration in the workplace is 22mg/m3 (USA).
3. Acute toxicity[16]
LD50: 121mg/kg (rat Oral); 890mg/kg (rabbit transdermal)
LC50: 29mg/m3 (rat inhalation)
4. Irritation Sex[17]
Rabbit transdermal: 524mg (24h), severe irritation.
Rabbit eye: 105mg, severe irritation.
5. Subacute and chronic toxicity[18] Feeding animals cresol can irritate the gastrointestinal tract, Corrosive effects can cause gastrointestinal bleeding, renal tubular damage, focal pneumonia, pulmonary congestion and liver cell necrosis.
6. Mutagenicity[19] Sister chromatid exchange: human fibroblasts 8mmol/L
Ecological data
1. Ecotoxicity[20]
LC50: 18~20.8mg/L (96h) ( Fish)
IC50: 6.8~33mg/L (72h) (algae)
2. Biodegradability[21]
Aerobic biodegradation (h): 24~168
Anaerobic biodegradation (h): 96~672
3. Non-biodegradability[22]
Aqueous phase photolysis half-life (h): 214~ 282
Photooxidation half-life in water (h): 66~3480
Photooxidation half-life in air (h): 1.6~16
Molecular structure data
1. Molar refractive index: 32.95
2. Molar volume (cm3/mol): 104.1
3. Isotonic specific volume (90.2K ): 259.9
4. Surface tension (dyne/cm): 38.8
5. Polarizability (10-24cm3): 13.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 70.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It may cause combustion when exposed to open flame, high heat or oxidants. It is slightly acidic and reacts with sodium hydroxide to form soluble sodium salt, but does not react with sodium carbonate. Sodium o-cresol reacts with an alkylating agent such as dimethyl sulfate to form phenol ether. Reacts with aldehydes to obtain synthetic resin. Catalytic hydrogenation produces methylcyclohexanol. Under mild conditions, o-cresol can undergo nitration, halogenation, alkylation and sulfonation reactions. O-cresol is easily oxidized and becomes darker when exposed to light and air, producing quinones and other complex compounds.
2. Toxic. It mainly affects the central nervous system and can even be fatal in severe cases. Cresol vapor or smoke can damage the skin and, when inhaled, can cause chronic nephritis and neurological disorders. The maximum allowable concentration in the air in the workplace is 5*10-6. Rat oral LD501350mg/kg. Production equipment should be sealed and operators should be equipped with protective equipment.
3. Stability[23] Stable
4. Incompatible substances[24] Strong oxidants, alkalis
5. Conditions to avoid contact[25] Light
6. Aggregation hazards[26] No aggregation
Storage method
Storage Precautions[27] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
The cresol fraction cut from the phenolic oil obtained by coal coking contains 6% o-cresol, 49.4% m-cresol, 32.1% p-cresol, xylenol and a small amount of phenol. The boiling point of o-cresol is 10°C lower than that of m- and p-cresol, and it is possible to separate it using a general distillation tower. The o-cresol fraction extracted from crude phenol distillation is distilled in a distillation column with no less than 40 theoretical plates. The reflux ratio is 14-15:1, and the distillation range is 188-192°C. The refined o-cresol obtained The melting point is about 29°C and the purity is greater than 97%. The synthesis methods mainly include the following:
1. Phenol alkylation method uses phenol and methanol as raw materials, and is obtained by methylation under a gauge pressure of about 4.14MPa and in the presence of an aluminum oxide catalyst. .

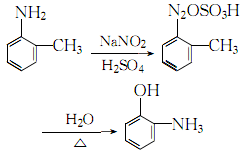
2. The o-toluidine method The method is used in reagent production and is obtained by diazotization and hydrolysis of o-toluidine.
3. Toluene hydroxylation method

4. Add o-toluidine to the sulfuric acid solution, stir and cool to obtain o-toluidine Toluidine sulfate, add ice cubes to cool down to 0~5?, maintain this temperature range, slowly add saturated sodium nitrite solution while stirring until the starch-potassium iodide test paper does not fade, which is the reaction end point. Add the obtained diazonium salt to concentrated sulfuric acid and stir thoroughly. Stop stirring. After the reactants react violently, add steam and heat until complete hydrolysis. Let stand and separate into layers, remove the oily layer, add 5% sodium hydroxide, filter out insoluble impurities, and then neutralize with 25% sulfuric acid until the pH value is 0. Leave to stand for layering, take the oil layer, and wash it twice with water. The crude product obtained is distilled under reduced pressure, and the 74-75.5°C fraction is collected at 1333 Pa to obtain the finished product. The process reaction formula is:
Purpose
1. Mainly used as synthetic resin. It can also be used to make pesticides, dimethyl tetrachloride herbicides, pharmaceutical disinfectants, spices, chemical reagents and antioxidants. Its downstream products mainly include synthetic resin o-cresol phenolic Resin, o-methyl salicylic acid, p-chloro-o-cresol, o-hydroxybenzaldehyde, 2-methyl-5-isopropylphenol and antioxidants, etc. In addition, it can also be used as diluent, disinfectant and plasticizer in the production of sebacic acid. It can also be used as important fine chemical intermediates such as synthetic pesticides, dyes, plastic antioxidants, polymerization inhibitors and spices.
2. Used as analytical reagents and in organic synthesis. [28]
extended-reading:https://www.newtopchem.com/archives/44412extended-reading:https://www.bdmaee.net/fascat-4200/extended-reading:https://www.newtopchem.com/archives/category/products/page/3extended-reading:https://www.bdmaee.net/dabco-t-45l-catalyst-cas121-143-5-evonik-germany/extended-reading:https://www.morpholine.org/dabco-ne1060-non-emissive-polyurethane-catalyst/extended-reading:https://www.bdmaee.net/nn-bis3-dimethylaminopropyl-nn-dimethylpropane-13-diamine/extended-reading:https://www.newtopchem.com/archives/category/products/page/160extended-reading:https://www.newtopchem.com/archives/44998extended-reading:https://www.newtopchem.com/archives/40329extended-reading:https://www.newtopchem.com/archives/39970