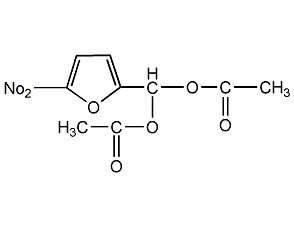
Structural formula
| Business number | 024W |
|---|---|
| Molecular formula | C9H9NO7 |
| Molecular weight | 243.17 |
| label |
5-Nitrofuryl methylene diethyl ester, 2-(acetylmethyl)-5-nitrofuran, 5-Nitrofurfural diacetyl acetal, 5-Nitro-2-furanmethandiol diacetate, Heterocyclic compounds |
Numbering system
CAS number:92-55-7
MDL number:MFCD00003244
EINECS number:202-166-1
RTECS number:LU1940000
BRN number:255089
PubChem ID:None
Physical property data
1. Properties: white crystalline powder or granules.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 90?92
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 52.09
2. Molar volume (cm3/mol): 174.1
3. Isotonic specific volume (90.2K ): 460.8
4. Surface tension (dyne/cm): 49.0
5. Polarizability (10-24cm3): 20.65
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 112
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 308
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain stereocenters of atoms: 0
13. The number of determined stereocenters of chemical bonds: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Low toxicity. Microsomal mutation test: Typhimurium 10?g/plate. See furfural.
?
Storage method
This product should be sealed and stored away from light.
Packaged in iron drums or plywood drums lined with plastic bags. Store in a cool, ventilated place. Moisture-proof and sun-proof. Store and transport according to general chemical product regulations.
Synthesis method
It is obtained by nitration and esterification of furfural. Add acetic anhydride to the reaction pot, cool it to -5°C, add 1/5 of the added amount of mixed acid (nitric acid and sulfuric acid) dropwise, and then add a mixture of nitric acid and furfural (1:2 volume) dropwise at the same time, and control the temperature at At about 0?, after adding it for about 5-6 hours, continue to add a part of furfural dropwise. Then, stir for 1 hour, add water and sodium carbonate solution, raise the temperature to 55-62°C, and keep it warm for 1 hour. Cool to 15°C, filter, wash with water, and spin dry to obtain the finished product, with a yield of 85%.
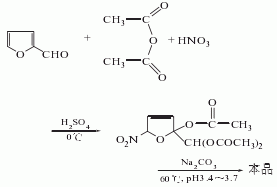
Purpose
It is a pharmaceutical intermediate, used in the preparation of furan anti-infective drugs (furatolin, nitrofuracillin, furantantine, etc.).
extended-reading:https://www.cyclohexylamine.net/lupragen-n203-teda-l33e/extended-reading:https://www.newtopchem.com/archives/44365extended-reading:https://www.bdmaee.net/bdmaee/extended-reading:https://www.cyclohexylamine.net/strong-gel-catalyst-dabco-dc1-delayed-strong-gel-catalyst/extended-reading:https://www.newtopchem.com/archives/945extended-reading:https://www.newtopchem.com/archives/38906extended-reading:https://www.cyclohexylamine.net/dabco-bl-17-niax-a-107-jeffcat-zf-54/extended-reading:https://www.bdmaee.net/2-hydroxypropyltrimethylammoniumformate/extended-reading:https://www.newtopchem.com/archives/45050extended-reading:https://www.bdmaee.net/di-n-butyldichlorotin/