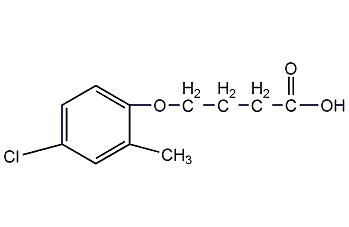
Structural formula
| Business number | 0287 |
|---|---|
| Molecular formula | C11H13ClO3 |
| Molecular weight | 228 |
| label |
2-Methyl-4-chlorobutyric acid, 2-Methyl-4-chlorophenoxybutyric acid, Bexone, Thitrol, Trifolex, herbicide |
Numbering system
CAS number:94-81-5
MDL number:MFCD00002820
EINECS number:202-365-3
RTECS number:ES8575000
BRN number:2215202
PubChem number:24862225
Physical property data
1. Properties: Crystalline solid.
2. Density (g/mL, 20?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 99?100
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, phenol, soluble in ethanol.
Toxicological data
1. Acute toxicity: Rat oral LD50: 680mg/kg; Mouse oral LD50: 800mg/kg;
2. Mutagenicity
Yeast gene conversion And mitotic recombination: 13500?mol/L;
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 58.11
2. Molar volume (cm3/mol): 185.8
3. Isotonic specific volume (90.2K ): 478.6
4. Surface tension (dyne/cm): 44.0
5. Polarizability (10-24cm3): 23.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 208
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
This product is produced by the reaction of 2-methyl-4-chlorophenol and ?-butyrolactone.
Purpose
Used as agricultural herbicide.
extended-reading:https://www.morpholine.org/category/morpholine/page/5401/extended-reading:https://www.bdmaee.net/strong-gel-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/23extended-reading:https://www.cyclohexylamine.net/main-9/extended-reading:https://www.newtopchem.com/archives/45013extended-reading:https://www.newtopchem.com/archives/40487extended-reading:https://www.bdmaee.net/fascat4201-catalyst-cas-818-08-6-dibutyl-tin-oxide/extended-reading:https://www.newtopchem.com/archives/45523extended-reading:https://www.bdmaee.net/chloriddi-n-butylcinicityczech/extended-reading:https://www.cyclohexylamine.net/pentamethyldipropene-triamine-cas-3855-32-1/