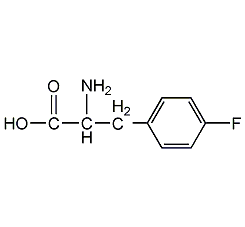
Structural formula
| Physical competition number | 0153 |
|---|---|
| Molecular formula | C9H10FNO2 |
| Molecular weight | 183.18 |
| label |
4-fluoro-DL-phenylalanine, 3-(4-Fluorophenyl)-DL-alanine, DL-Phe(p-F), H-DL-Phe(p-F)-OH |
Numbering system
CAS number:51-65-0
MDL number:MFCD00002600
EINECS number:200-113-7
RTECS number:AY6315000
BRN number:29338793
PubChem number:24894885
Physical property data
1. Properties: white crystal.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 263~264? (253~255?) decomposes.
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition Combustion temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in acetic acid and alkali, about 10mg of product can be dissolved in 1ml of water.
Toxicological data
1. Acute toxicity: mouse abdominal cavity LD50: >1mg/kg
4. Mutagenicity: Mutation microorganismsTEST system: Microorganisms – not otherwise specified: 50umol/L; Mutation microorganismsTEST system: mold – Neurospora crassa: 1mg /L; Gene conversion and mitotic recombinationTEST system: yeast-Saccharomyces cerevisiae: 1200ppm; Sex chromosome loss and nondisjunctionTEST system: yeast-Saccharomyces cerevisiae: 1200ppm; Cytogenetic analysisTEST system: Human lymphocytes: 50umol/L; Cytogenetic analysisTEST System: rodent-hamster lung: 550umol/L; DNA inhibitionTEST system: rodent-rabbit kidney: 500umol/L
Ecological data
None
Molecular structure data
1. Molar refractive index: 45.48
2. Molar volume (cm3/mol): 141.6
3. Isotonic specific volume (90.2K): 378.9
4. Surface tension (dyne/cm): 51.2
5. Polarizability (10-24cm3): 18.03
p>
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 63.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 179
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Should be kept sealed.
Synthesis method
None
Purpose
E. coli growth inhibitor. Phenylalanine antagonist. Protein synthesis inhibitor.
extended-reading:https://www.cyclohexylamine.net/2-2-dimethylaminoethylmethylaminoethanol/extended-reading:https://www.newtopchem.com/archives/44441extended-reading:https://www.newtopchem.com/archives/44735extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/10/149.jpgextended-reading:https://www.newtopchem.com/archives/43964extended-reading:https://www.newtopchem.com/archives/39820extended-reading:https://www.newtopchem.com/archives/44097extended-reading:https://www.bdmaee.net/teda-l33e-polyurethane-amine-catalyst-tosoh/extended-reading:https://www.newtopchem.com/archives/category/products/page/13extended-reading:https://www.newtopchem.com/archives/category/products/page/47