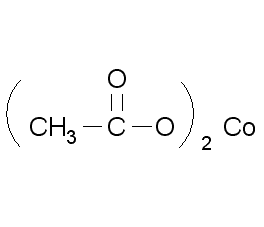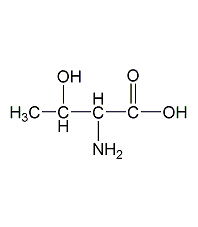
Structural formula
| Business number | 01GX |
|---|---|
| Molecular formula | C4H9NO3 |
| Molecular weight | 119.12 |
| label |
L-Hydroxybutyrate, (2S,3R)-2-amino-3-hydroxybutyric acid, L-?-amino-?-hydroxybutyric acid, L-isoerythora amino acid, (2S,3R)-2-Amino-3-hydroxybutyric acid, a-Amino-?-hydroxybutyric acid, nutritional additives, intermediates, Biochemical reagents |
Numbering system
CAS number:72-19-5
MDL number:MFCD00064270
EINECS number:200-774-1
RTECS number:XO8590000
BRN number:1721646
PubChem number:24900544
Physical property data
1. Properties: white crystal or crystalline powder, containing 1/2 crystal water. Odorless, slightly sweet taste. 2. Density (g/mL, 25/4?): 1.307
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 256 (dec.)(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: -28 ° (C=6, H2O)
8. Flash point (ºC): Uncertain
9. Specific rotation (º) : -28.4 º (c=6, H2O)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25 ºC): Uncertain
12. Saturated vapor pressure (kPa, 60 ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14 . Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19 . Solubility: Easily soluble in water (200g/L, 25?), insoluble in methanol, ethanol, ether and chloroform.
Toxicological data
Acute toxicity: rat abdominal LD50: 3098 mg/kg;
Mutagenicity: human lymphocyte Sister chromatid exchangeTEST SYSTEM: 10 mg/L;
Ecological data
None
Molecular structure data
1. Molar refractive index: 27.13
2. Molar volume (cm3/mol): 91.1
3. Isotonic specific volume (90.2K ): 253.6
4. Surface tension (dyne/cm): 60.0
5. Polarizability (10-24cm3): 10.75
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -2.9
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
p>
4. Number of rotatable chemical bonds: 2
5. Topological molecular polar surface area (TPSA): 83.6
6. Number of heavy atoms: 8
7. Surface charge: 0
8. Complexity: 93.3
9. Number of isotope atoms: 0
10. Determine the atomic stereocenter Number: 2
11. Number of uncertain atomic stereocenters: 0
12. Number of determined chemical bond stereocenters: 0
13. Uncertain chemical bonds Number of stereocenters: 0
14, Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves and smoke.
Storage method
Tightly packaged in brown wide-mouth glass bottles. Store in a cool, dry place away from light.
Synthesis method
1. It is produced by fermenting sugar, ammonia and homoserine with glutamic acid-producing Pediococcus, Brevibacterium, Corynebacterium, etc., and then refining it. The concentration in the fermentation broth can reach 5-20g/L.
2.Prepared from crotonic acid or glycine as raw material. Glucose or starch can also be used as raw materials, and the product can be obtained through fermentation and refining using Escherichia coli, Brevibacterium flavum, Bacillus glutamicum or Corynebacterium craniodonta as strains.
3.L-The industrial production methods of threonine mainly include: ? synthesis method; ? fermentation method; ? protein hydrolysis method Three types. Fermentation production is now advocated.
The fermentation method uses sugar, ammonia, and homoserine (homoserine) as the culture medium, and is fermented with glutamic acid-producing Pediococcus (glutaminus) and refined to produce L-threonine.
4.Extraction method
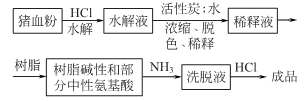
5.The injection preparation method is as follows

6. Tobacco: BU, 22; FC, 21; Synthesis: It can be obtained by hydrolysis and purification of proteins (such as casein), or synthesized from methanol and mercury acetate in acrylic acid derivatives.
Purpose
1. Mainly used as nutritional supplement. When heated with glucose, it easily generates caramel and chocolate aromas, and has a flavor-enhancing effect. Can also be used for biochemical research.
2.As a feed nutritional fortifier, threonine is an essential amino acid. Threonine is often added to the feed of immature piglets and poultry, and is the second limiting amino acid in pig feed and the third limiting amino acid in poultry feed. Added to feeds based on wheat, barley and other grains.
3.Nutritional additives, also used to prepare amino acid infusions and comprehensive amino acid preparations.
4.For the auxiliary treatment of peptic ulcer. It can also treat anemia and cardiovascular system diseases such as angina pectoris, aortitis, and cardiac insufficiency.
extended-reading:https://www.bdmaee.net/cas2212-32-0/extended-reading:https://www.bdmaee.net/nt-cat-bdmaee/extended-reading:https://www.morpholine.org/dimethylethanolamine/extended-reading:https://www.morpholine.org/category/morpholine/page/5390/extended-reading:https://www.cyclohexylamine.net/high-quality-cas-26761-42-2-potassium-neodecanoate/extended-reading:https://www.newtopchem.com/archives/category/products/page/6extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-R-8020-Jeffcat-TD-20-TEDA-A20.pdfextended-reading:https://www.newtopchem.com/archives/999extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/1-4.jpgextended-reading:https://www.newtopchem.com/archives/44906