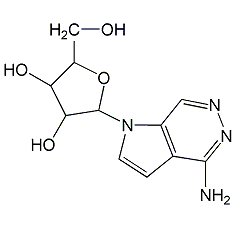
Structural formula
| Business number | 01FM |
|---|---|
| Molecular formula | C11H14N4O4 |
| Molecular weight | 265.25 |
| label |
7-Deazadenosine, 7-.beta.-D-ribofuranosyl-7H-pyrrolo-[2,3-d]pyrimidin-4-amine, 4-Amino-7.beta.-D-ribofuranosyl-7H-pyrrolo(2,3-d)pyrimidine, Genetic engineering research reagents |
Numbering system
CAS number:69-33-0
MDL number:MFCD00056012
EINECS number:200-703-4
RTECS number:UY8870000
BRN number:38498
PubChem number:24899902
Physical property data
1. Character:White needle crystal
2. Density (g/mL,25/4?): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):247?248? (decomposition)
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º):-67 º, c=1,50%acetic acid
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturated vapor pressure (kPa,60ºC): Unsure
13. Heat of combustion (KJ/mol): Unsure
14. Critical temperature (ºC): Unsure
15. Critical pressure (KPa): Unsure
16. Oil and water (octanol/Log value of water) partition coefficient: Uncertain
17. Explosion limit (%,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:1gsoluble in330mlWater, 200mlMethanol and2000mlIn ethanol, insoluble in acetone , ethyl acetate, chloroform, benzene and petroleum ether
Toxicological data
Acute toxicity: Rat oral LD50: 16 mg/kg; Rat abdominal cavity LD50 : 1 mg/kg;
Mouse oral LD50: 28320 ug/kg; mouse abdominal cavity LD50: 6 mg/kg;
Mouse veinLD50: 45 ug/kg; Dog oral LDLo?48 mg/kg; Dog VeinLDLo: 48 mg/kg;
Mutagenic: mouse leukocytesDNA Suppress test system:5400 ug/L;Mouse leukocyte change test system: 290 ug/L;
??Mouse abdominal cavityDominant lethal test?500 ug/kg;Rabbit KidneyDNA Suppression test system:18ug/L;
Rabbit KidneyDNAChange test system (test system not unless otherwise specified):30 ug/L;
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 61.51
2. Molar volume (m3/mol??139.5
3. isotonic specific volume (90.2K):431.6
4. Surface Tension (dyne/cm):91.5
5. Polarizability?10-24cm3): 24.38
Compute chemical data
1. Hydrophobic parameters Calculate reference value (XlogP??-1.3
2. Hydrogen Bonding Number of donors: 4
3. Hydrogen Bonding Number of receptors: 7
4. Rotatable Number of chemical bonds: 2
5. Interchange Number of isomers: 4
6. Topological molecular polarity Surface area (TPSA): 127
7. Heavy atoms Quantity: 19
8. Surface charge ?0
9. Complexity ?334
10. Isotope atomic number:0
11. Determine the number of atomic stereocenters:4
12. Uncertain number of atomic stereocenters:0
13. Determine the number of stereocenters of chemical bonds:0
14. Uncertain number of chemical bond stereocenters:0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
None
Purpose
To Mycobacterium tuberculosisBCGMinimum inhibitory concentration MICLess than1?g/ml, 5?g/mlSuppress miceNFSarcoma cells, inhibit sarcoma in animals180, Ehrlich ascites carcinoma is also responsible for Pyroplasma oryzae and Candida albicans There is a weak inhibitory effect.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Niax-Catalyst-A-1-MSDS.pdfextended-reading:https://www.bdmaee.net/lupragen-n204-catalyst-dimethylpiperazine-basf/extended-reading:https://www.bdmaee.net/cas-2273-45-2/extended-reading:https://www.bdmaee.net/kaolizer-12/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/di-n-butyl-tin-diisooctoate-CAS2781-10-4-FASCAT4208-catalyst.pdfextended-reading:https://www.bdmaee.net/nt-cat-tmpda-catalyst-cas10294-43-5-newtopchem/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/102-8.jpgextended-reading:https://www.newtopchem.com/archives/44765extended-reading:https://www.bdmaee.net/dibutyl-tin-oxide-food-grade/extended-reading:https://www.bdmaee.net/cas-2781-10-4/