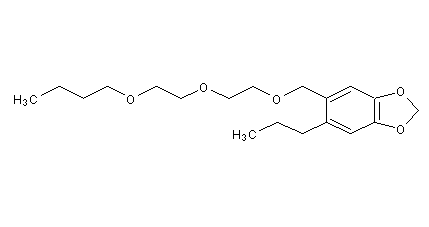
Structural formula
| Business number | 014D |
|---|---|
| Molecular formula | C19H30O5 |
| Molecular weight | 338.44 |
| label |
Piperonyl butoxide, 3,4-methylenedioxy-6-(butoxyethoxyethoxymethyl)propylbenzene, 5-(2-(2-butoxyethoxy)ethoxymethyl)-6-propyl-1,3-benzodioxolane, a-[2-(2-Butoxyethoxy)ethoxy]-4,5-methylenedioxy-2-propyltoluene, 6-Propylpiperonylbutyldiethylene glycol ether, 5-[[2-(Butoxyethoxy)ethoxy]methyl]-6-propyl-1,3-benzodioxole, Synergist |
Numbering system
CAS number:51-03-6
MDL number:MFCD00005842
EINECS number:200-076-7
RTECS number:XS8050000
BRN number:288063
PubChem number:24869144
Physical property data
1. Properties: Colorless and odorless liquid.
2. Density (g/mL, 25/4?): 1.055
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 1.33kPa): 180( 133.3pa)
7. Refractive index (n20D): 1.50
8 . Flash point (ºC): 171
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/ mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water ( Log value of the partition coefficient (octanol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/ V): Undetermined
19. Solubility: Miscible with methanol, ethanol, benzene, Freon and other organic solvents.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 93.81
2. Molar volume (cm3/mol): 317.3
3. Isotonic specific volume (90.2K ): 793.1
4. Surface tension (dyne/cm): 38.9
5. Polarizability (10-24cm3): 37.18
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 13
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 46.2
7. Number of heavy atoms: 24
8. Surface charge: 0
9. Complexity: 312
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exist in smoke.
2. Large doses can cause vomiting and diarrhea.
Storage method
None
Synthesis method
It is produced by catalytic hydrogenation and reduction of safrole, chloromethylation with formaldehyde and hydrochloric acid, and etherification with butoxyethoxyethanol. Broad spectrum synergist.
Purpose
It can improve the insecticidal activity of pyrethrins, various pyrethroids, rotenone and carbamate insecticides.
extended-reading:https://www.newtopchem.com/archives/466extended-reading:https://www.cyclohexylamine.net/polyurethane-tertiary-amine-catalyst-catalyst-25-s/extended-reading:https://www.bdmaee.net/dimethylbis1-oxoneodecyloxystannane/extended-reading:https://www.newtopchem.com/archives/44625extended-reading:https://www.bdmaee.net/tributyltin-chloride-cas1461-22-9-tri-n-butyltin-chloride/extended-reading:https://www.newtopchem.com/archives/category/products/page/161extended-reading:https://www.morpholine.org/n-3-dimethyl-amino-propyl-n-n-diisopropanolamine/extended-reading:https://www.bdmaee.net/dabco-dmaee-catalyst-cas1704-62-7-evonik-germany/extended-reading:https://www.bdmaee.net/niax-k-zero-3000-trimer-catalyst-momentive/extended-reading:https://www.bdmaee.net/dabco-tmeda-catalyst-cas-110-18-9-evonik-germany/