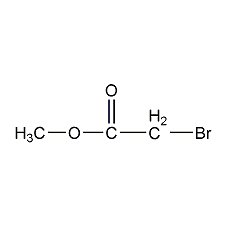
Structural formula
| Business number | 02AY |
|---|---|
| Molecular formula | C3H5BrO2 |
| Molecular weight | 152.97 |
| label |
Methyl bromoacetate, Bromo acetic acid methyl ester, pesticides, Mildew remover solvent, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:96-32-2
MDL number:MFCD00000189
EINECS number:202-499-2
RTECS number:AF6300000
BRN number:506256
PubChem number:24850306
Physical property data
1. Properties: colorless to yellow liquid, hygroscopic. [1]
2. Melting point (?): -50[2]
3. Boiling point (?): 145~146.7[3]
4. Relative density (water=1): 1.635 (20?)[4]
5. Saturated vapor pressure (kPa): 2.00 (51?)[5]
6. Octanol/water partition coefficient: 0.72[6]
7. Flash point (?): 62.8[7]
8. Solubility: insoluble in water, soluble in methanol and ether . [8]
Toxicological data
1. Acute toxicity: Intravenous injection of LCL0 into mice: 15800?g/kg;
2. It is strongly irritating to the eyes, mucous membranes or skin, has tear-inducing properties in the eyes, and can cause severe burns. It is easy to be absorbed through the skin if it touches the skin, or it may cause poisoning if accidentally ingested or inhaled vapor or dust. The maximum allowable concentration in the air is 100mg/m3.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 25.45
2. Molar volume (cm3/mol): 94.7
3. Isotonic specific volume (90.2K ): 228.3
4. Surface tension (dyne/cm): 33.7
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 10.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 52.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Decomposition by heat releases toxic gases. Can burn when exposed to open fire.
2. Stability[9] Stable
3. Incompatible substances[10] Acids, alkalis, strong oxidants, strong reducing agents
4. Avoid contact? Conditions[11] Heating
5. Polymerization hazard[12] No polymerization p>
6. Decomposition products[13] Hydrogen bromide
Storage method
Storage Precautions[14] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30°C and the relative humidity should not exceed 70%. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency spill treatment equipment and suitable containment materials.
Synthesis method
1. Obtained from bromination of acetic acid and then esterification with methanol. Add glacial acetic acid, acetic anhydride, red phosphorus and several pieces of zeolite into the dry reactor, heat to reflux, and when the temperature rises to 60-70°C, start slowly adding bromine dropwise, and keep it warm for about 1 hour after the addition. Cool and filter, then distill under reduced pressure to obtain bromoacetic acid. Add bromoacetic acid and methanol to carbon tetrachloride, then add sulfuric acid, heat to reflux and dehydrate. After dehydration is completed, steam out the carbon tetrachloride, then add methanol and reflux for 1 hour to recover the methanol. The residue is washed, dried and decompressed. Distillation, that is, the finished product is obtained.
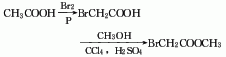
2. In a dry place A condenser, thermometer, separatory funnel, etc. are installed on the flask. Add glacial acetic acid, acetic anhydride, red phosphorus and a few pieces of zeolite, and then reflux on the sand bath. When the temperature reaches 60~70°C, start slowly dripping bromine from the separatory funnel. After adding, keep it warm for 30~60 minutes. Cool and filter, then distill under reduced pressure to obtain bromoacetic acid. Mix carbon tetrachloride, bromoacetic acid, methanol, and sulfuric acid on a sand bath and reflux and dehydrate. After dehydration is completed, the carbon tetrachloride is evaporated, and then methanol is added and refluxed for 1 hour. The methanol is recovered. After the residue is washed and dried, reduce the Pressure distillation to obtain the finished product. The process reaction formula is:
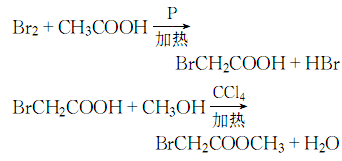
Purpose
Used in the synthesis of herbicides, and also used as intermediates in the manufacture of dyes and pharmaceuticals. [15]
extended-reading:https://www.morpholine.org/category/morpholine/page/4/extended-reading:https://www.newtopchem.com/archives/category/products/page/141extended-reading:https://www.morpholine.org/nn-bis3-dimethylaminopropyl-nn-dimethylpropane-13-diamine/extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Niax-A-99-MSDS.pdfextended-reading:https://www.bdmaee.net/dibutyltin-didodecanoate/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Catalyst-PT303-PT303-polyurethane-catalyst-PT303.pdfextended-reading:https://www.bdmaee.net/22-dimorpholinodiethylether-3/extended-reading:https://www.cyclohexylamine.net/delay-catalyst-a-300-amine-catalyst-a-300/extended-reading:https://www.bdmaee.net/anhydrous-tin-tetrachloride-cas-7646-78-8-tin-tetrachloride/extended-reading:https://www.newtopchem.com/archives/44797