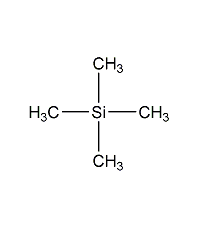
Structural formula
| Business number | 01K1 |
|---|---|
| Molecular formula | C4H12Si |
| Molecular weight | 88.22 |
| label |
Tetramethylsilane, Tetramethylsilane, tetramethyl silicon, Silicon Tetramethyl, Tetramethylsilicane, Si(CH3)4, inorganic solvents, NMR reagents |
Numbering system
CAS number:75-76-3
MDL number:MFCD00008274
EINECS number:200-899-1
RTECS number:None
BRN number:1696908
PubChem number:24889006
Physical property data
1. Properties: colorless hygroscopic liquid, easily volatile. [1]
2. Melting point (?): -99[2]
3. Boiling point (?): 26~27[3]
4. Relative density (water=1): 0.65 (20?)[4]
5. Saturated vapor pressure (kPa): 80.3 (20?)[5]
6. Critical pressure (MPa): 2.81[6]
7. Octanol/water partition coefficient: 3.24[7]
8. Flash point (?): -27.22[ 8]
9. Ignition temperature (?): 450[9]
10. Explosion upper limit (%): 37.9[10]
11. Lower explosion limit (%): 1[11]
12. Solubility: insoluble in water and cold concentrated sulfuric acid, soluble in most organic solvents such as ether. [12]
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
3. Subacute and chronic toxicity [13] Rats (4 males) inhaled vapor 3.6g/m3, 6 hours, 15 times, resulting in lethargy and organ congestion.
Ecological data
Not harmful to water.
Molecular structure data
1. Molar refractive index: 29.29
2. Molar volume (cm3/mol): 130.2
3. Isotonic specific volume (90.2K ): 250.4
4. Surface tension (dyne/cm): 13.6
5. Polarizability (10-24cm3): 11.61 p>
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 19.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemically stable, not concentratedDecomposed by sulfuric acid or strong alkali. Under ultraviolet irradiation, it can be chlorinated into chloromethyltrimethylsilane. Tetramethylsilane has high thermal stability and only begins to decompose at 660 to 720°C.
2. Stability[14] Stable
3. Incompatible substances[15] Strong oxidants, strong acids, strong bases
4. Avoid humid conditions [16] Humid air
5. Hazards of aggregation[17] No aggregation
Storage method
Storage Precautions[18] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29?. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It can be prepared by reacting tetrachlorosilane or ethyl orthosilicate with methylmagnesium iodide. It can also be prepared by reacting methyl chloride and silicon powder in the presence of copper catalyst. It is produced by reacting at high temperature and then carefully fractionating it. Since silicon has low electronegativity and has little effect on the hydrogen atoms on the four methyl groups, it can give a strong signal and a sharp absorption peak in the nuclear magnetic resonance spectrum, while the protons in other general organic compounds The absorption peaks all appear to its left. Therefore, TMS is usually used as the internal standard for chemical shifts in NMR spectra, and its chemical shift is set to zero.
Purpose
1. Used as reagents, aviation fuel, solvents, and nuclear magnetic resonance reagents.
2. Used as reagents and aviation fuel. [19]
extended-reading:https://www.newtopchem.com/archives/44034extended-reading:https://www.bdmaee.net/niax-b-9-balanced-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/44716extended-reading:https://www.bdmaee.net/anhydrous-tin-chloride/extended-reading:https://www.cyclohexylamine.net/tmr-2-cas-62314-25-4-2-hydroxypropyltrimethylammoniumformate/extended-reading:https://www.bdmaee.net/dibutyltin-diacetate/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyltin-acetate-CAS1067-33-0-tributyltin-oxide.pdfextended-reading:https://www.newtopchem.com/archives/535extended-reading:https://www.bdmaee.net/22-dimorpholinodiethylether-2/extended-reading:https://www.morpholine.org/dabco-33-s-microporous-catalyst/