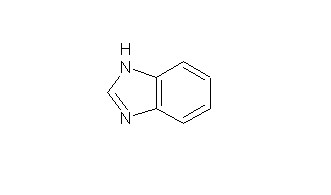
Structural formula
| Business number | 014H |
|---|---|
| Molecular formula | C7H6N2 |
| Molecular weight | 118 |
| label |
1,3-Benzodiazole, 1H-benzimidazole, benzodiazole, Metadiazine, Benzimidazole, benzoxine, 1H-Benzimidazole, N,N’-Methylenyl-o-phenylenediamine, Azindole, Heterocyclic compounds |
Numbering system
CAS number:51-17-2
MDL number:MFCD00005585
EINECS number:200-081-4
RTECS number:DD5425000
BRN number:109682
PubChem number:24847491
Physical property data
1. Properties: white orthorhombic or monoclinic crystal. Has good chemical stability.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
>
4. Melting point (ºC): 170.5
5. Boiling point (ºC, normal pressure): >360
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in hot water, ethanol, boiling xylene, acid and strong alkali aqueous solution, slightly soluble in cold water and ether, almost insoluble in benzene and petroleum ether .
Toxicological data
1. Acute toxicity: rat oral LDLo: 500mg/kg; rat abdominal LD50: 385mg/kg; mouse oral LD50: 2910mg/kg; mouse abdominal LD50: 445mg/kg; mouse intravenous LD50: 280mg/ kg2, mutagenicity: mutation microorganismsTEST system: bacteria – Salmonella typhimurium: 250ug/plate; mutation microorganismsTEST system: bacteria – Escherichia coli: 1mg/disc; DNA damageTEST system: bacteria – Escherichia coli: 15mmol/L/48H; phage Inhibit capacityTEST system: Bacteria – E. coli: 1gm/L
Ecological data
None
Molecular structure data
1. Molar refractive index: 36.61
2. Molar volume (cm3/mol): 95.0
3. Isotonic specific volume (90.2K ): 264.8
4. Surface tension (dyne/cm): 60.1
5. Polarizability (10-24cm3): 14.51
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 28.7
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 103
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place. 25kg bag or barrel, lined with plastic bag. ?
Synthesis method
Originated from the cyclization of o-phenylenediamine and formic acid. Heat the mixture of o-phenylenediamine and formic acid on a water bath for 2 hours, cool it, adjust the pH to 10 with 10% sodium hydroxide solution, filter out the precipitated solid, and wash it with cold water to obtain a crude product. Add water to slightly boil the crude product, add activated carbon to decolorize, filter while hot, cool the filtrate to room temperature, filter, wash with cold water, and dry at 100°C to obtain the finished product. The crude product yield is 90%.
The median lethal dose (mice, oral) is 2190mg/kg.
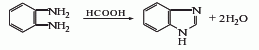
Purpose
Reagent for testing cobalt. ?
extended-reading:https://www.bdmaee.net/fomrez-sul-11a-catalyst-momentive/extended-reading:https://www.morpholine.org/category/morpholine/page/2/extended-reading:https://www.newtopchem.com/archives/987extended-reading:https://www.bdmaee.net/polycat-31-polyurethane-spray-catalyst-polycat-31-hard-foam-catalyst-polycat-31/extended-reading:https://www.newtopchem.com/archives/674extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/59.jpgextended-reading:https://www.newtopchem.com/archives/1817extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Niax-A-1-MSDS.pdfextended-reading:https://www.newtopchem.com/archives/44177extended-reading:https://www.morpholine.org/high-quality-cas-26761-42-2-potassium-neodecanoate/