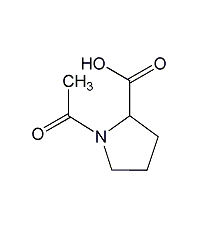
Structural formula
| Business number | 01FF |
|---|---|
| Molecular formula | C7H11NO3 |
| Molecular weight | 157.18 |
| label |
N-Acetyl-L-proline, 1-Acetyl-L-proline, Acetyl-L-proline, Nalpha-Acetyl-L-proline |
Numbering system
CAS number:68-95-1
MDL number:MFCD00020837
EINECS number:200-698-9
RTECS number:None
BRN number:83200
PubChem number:24890453
Physical property data
1. Appearance: White needle-like crystal
2. Density (g/mL, 25/4?): Uncertain
3. Relative vapor density (g/mL , air=1): Uncertain
4. Melting point (ºC): 115-117
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): -86 º (c=1 EtOH)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor Pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
p>
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil-water (octanol/water) partition coefficient The logarithmic value of p>
19. Solubility: Uncertain
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 37.32
2. Molar volume (cm3/mol): 123.2
3. Isotonic specific volume (90.2K ): 332.3
4. Surface tension (dyne/cm): 52.8
5. Polarizability (10-24cm3): 14.79
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 57.6
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 190
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be stored in a sealed, cool, dry place.
Synthesis method
None
Purpose
It is an important fine organic chemical intermediate and is widely used in medicine, pesticides, chemical industry and other fields.
extended-reading:https://www.newtopchem.com/archives/category/products/page/101extended-reading:https://www.newtopchem.com/archives/40538extended-reading:https://www.bdmaee.net/cas-4394-85-8/extended-reading:https://www.newtopchem.com/archives/100extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/1-6.jpgextended-reading:https://www.bdmaee.net/cas-33568-99-9/extended-reading:https://www.bdmaee.net/cas-2273-45-2/extended-reading:https://www.bdmaee.net/toyocat-et-catalyst-tosoh/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/75.jpgextended-reading:https://www.newtopchem.com/archives/1126