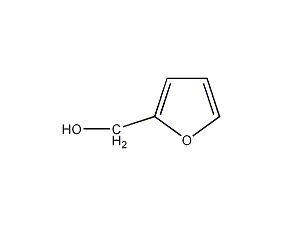
Structural formula
| Business number | 02D1 |
|---|---|
| Molecular formula | C5H6O2 |
| Molecular weight | 98.10 |
| label |
?-furanmethanol, 2-furanmethanol, Glutol, 2-Hydroxymethylfuran, Oxocene methanol, ?-Furan methanol, 2-Furfurylalcohol, Gluten alcohol, 2-Hydroxymethyl-furan, Fruylcarbinol, heterocyclic compounds, synthetic raw materials |
Numbering system
CAS number:98-00-0
MDL number:MFCD00003252
EINECS number:202-626-1
RTECS number:LU9100000
BRN number:106291
PubChem number:24851190
Physical property data
1. Properties: colorless or light yellow liquid, which turns brown or dark red when exposed to sunlight and air, and has a special bitter and spicy smell.
2. Boiling point (ºC, 101.3kPa): 170.0
3. Melting point (ºC, freezing point, quasi-stable state): -14.6
4. Melting point (ºC, freezing point, stable state): -14.63
5. Relative density (g/mL, 20/4ºC): 1.1285
6. Relative density (g/mL, 30 /4ºC): 1.12384
7. Relative vapor density (g/mL, air=1): 3.4
8. Refractive index (20ºC): 1.4868
9. Refractive index (30ºC): 1.4801
10. Viscosity (mPa·s, 25ºC): 4.62
11. Flash point (ºC, closed): 65
12. Flash point (ºC): 391
13. Heat of evaporation (KJ/mol, 25ºC): 50
14. Heat of evaporation (KJ/mol, b.p.) :53.6
15. Heat of fusion (KJ/mol): 13.138
16. Heat of formation (KJ/mol): 276.54
17. Heat of combustion ( KJ/mol, standard conditions): 2550.43
18. Specific heat capacity (KJ/(kg·K), 26.8ºC, constant pressure): 2.09
19. Vapor pressure (kPa, 25ºC): 0.08
20. Lower explosion limit (%, V/V): 1.8
21. Upper explosion limit (%, V/V): 16.3
22. Solubility: Easily soluble in water, ethanol, ether, acetone, ethyl acetate and other organic solvents, but insoluble in non-polar organic solvents such as paraffin. Can dissolve grease, natural resin, cellulose acetate, ethyl cellulose, nitrocellulose, polyvinyl acetate, polymethylmethacrylate, etc.
23.Solubility parameter (J·cm-3)0.5: 25.089
24. van der Waals area (cm2 ·mol-1): 6.650×109
25. van der Waals volume (cm3·mol-1): 51.190
26. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2613.2
27. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -211.8
28. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2548.8
29. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -276.2
30. Liquid phase standard entropy (J·mol-1·K-1): 215.47
31. Liquid phase standard free energy of formation (kJ·mol-1): -178.2
32. Liquid phase standard hot melt (J·mol-1·K– 1): 202.8
Toxicological data
1. Acute toxicity: Oral – rat LD50: 275 mg/kg; Oral – mouse LC50: 160 mg/ Kilogram 2. Toxicity Classification: Highly toxic
3. Irritation data: Eyes – Rabbit 100 mg/24 hours Moderate 4. Furfuryl alcohol is a moderately toxic substance and is highly irritating to the eyes. Small doses can stimulate the breathing of humans and rabbits, while larger doses can inhibit breathing and lower body temperature, causing nausea, dizziness, salivation, diarrhea and diuresis. The maximum allowable concentration in the workplace is 200 mg/m3.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 25.00
2. Molar volume (cm3/mol): 86.0
3. Isotonic specific volume (90.2K): 214.7
4. Surface tension (dyne/cm): 38.8
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 9.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 33.4
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 54
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with air. Avoid contact with acid chloride, oxygen, and acids. 2. Colorless and easy-flowing liquid, it will turn brown or dark red when exposed to sunlight or air. Has a bitter taste. Miscible with water, but unstable in water, easily soluble in ethanol, ether, benzene and chloroform, but insoluble in petroleum hydrocarbons. Insoluble in alkanes. It easily polymerizes and explodes violently when exposed to acid. It is flammable. Steam and air can form an explosive mixture, with an explosion limit of 1.8%-16.3% (volume fraction). It is stable to alkali. When adding alkaline substances such as tripropylamine, it can prevent the tendency of auto-oxidation. Moderately toxic. Furfuryl alcohol undergoes auto-oxidation under the action of oxygen in the air and turns brown, and the moisture content and acidity also increase. Adding alkaline substances such as tripropylamine can prevent the auto-oxidation of furfuryl alcohol. Furfuryl alcohol reacts with strong inorganic acids or strong organic acids to cause explosion. Avoid being close to strong acids during storage. The flammability of furfuryl alcohol is similar to that of kerosene. It should be kept away from water sources. In case of fire, use carbon dioxide or powder fire extinguishing agent to extinguish the fire.
3. Chemical properties: Furfuryl alcohol can reduce the ammonia solution of silver nitrate when heated. It is stable to alkali, but prone to resinization under the action of acid or oxygen in the air. It is particularly sensitive to strong acids and often catches fire when reacting violently. Blue color appears when heated with a mixture of diphenylamine, acetic acid, and concentrated sulfuric acid (diphenylamine reaction).
4. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
Storage method
It should be sealed away from light and stored in a cool place. Avoid close contact with strong acids during storage. It should be kept away from water sources and can be stored in iron, mild steel or aluminum containers.
Synthesis method
1. Refining method: dry with anhydrous sodium sulfate or anhydrous potassium carbonate and then fractionate. Fractionation is best carried out under a stream of nitrogen. You can also perform vacuum distillation first to remove tar-like substances, then shake it with sodium bisulfite aqueous solution, dry it with anhydrous sodium sulfate, and then fractionate it under reduced pressure in the presence of sodium carbonate.
2. The industrial production method of furfuryl alcohol is produced by hydrogenation of furfural:
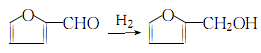
Hydrogenate into liquid There are two types of phase hydrogenation and gas phase hydrogenation. Liquid phase hydrogenation method: Furfural and hydrogen exist in a Cu-Cr-Ca catalyst in a ratio of 1:42 (molar ratio)The reaction is carried out at 190~210?, 5-8MPa (or above 10MPa, 170?, using Cu-Cr catalyst). After the reaction is completed, settle and remove the solid catalyst. The resulting liquid is crude furfuryl alcohol. Gas-phase hydrogenation method: In a tubular reactor, furfural and hydrogen are reacted at 1:42 (molar ratio) in the presence of a Ni-Cu or Cr-Cu catalyst at 80 to 170°C and 0.1 to 0.39MPa. have to. The obtained crude furfuryl alcohol is rectified under reduced pressure at 80-87kPa to remove tar-like substances, then washed with sodium bisulfite, dried and dehydrated, and then sodium carbonate is added to reduce the pressure. Distill to obtain pure furanmethanol.
3.The disproportionation method uses furfural as raw material to cause a disproportionation reaction of furfural in the presence of caustic soda. The advantage is that the equipment is simple and no reducing agent is required. The disadvantage is that the utilization rate of raw materials is low.
4. Tobacco: BU, 56; OR, 18; FC, 9; FC, BU, OR, 18; FC, 18; FC, 40.
Purpose
1. In addition to being used as a raw material for furan resin, furfuryl alcohol can also be used as a dye, solvent, dispersant, and wetting agent for varnish, phenolic resin, and furan resin. The plasticizer made from it has better cold resistance than the esters of butanol and octanol. 2.Used for synthetic resin. Used as solvent and preservative for dyes and resins. 3.is an important chemical and light industrial raw material, used to synthesize furfural resins with various properties, adhesives in the machine-made casting industry, furan resins or Adhesives, urea-formaldehyde resin, phenolic resin, furfuryl alcohol-urea-formaldehyde resin, synthetic rubber, pesticides, cold-resistant plasticizers, etc. It is also used as a solvent, diluent, modifier, dispersant or wetting agent for coatings, dyes, furan resins, furfural resins, etc. It is also used as a rocket fuel additive and paint stripper. 3. Used in organic synthesis, synthetic fibers, rubber, pesticides, etc.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/72.jpgextended-reading:https://www.newtopchem.com/archives/1875extended-reading:https://www.bdmaee.net/tris-dimethylaminopropyl-hexahydrotriazine-cas-15875-13-5-triazine-catalyst/extended-reading:https://www.newtopchem.com/archives/44045extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/88-1.jpgextended-reading:https://www.bdmaee.net/anhydrous-tin-chloride/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/3-7.jpgextended-reading:https://www.cyclohexylamine.net/category/product/page/2/extended-reading:https://www.bdmaee.net/high-quality-tris3-dimethylaminopropylamine-cas-33329-35-0-nn-bis3-dimethylaminopropyl-nn-dimethylpropane-13-diamine/extended-reading:https://www.bdmaee.net/tegoamin-bde-catalyst-cas121-54-0-degussa-ag/