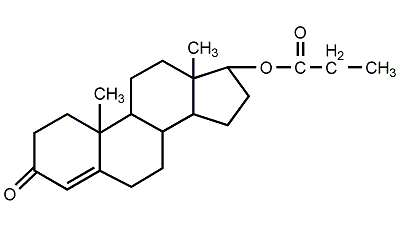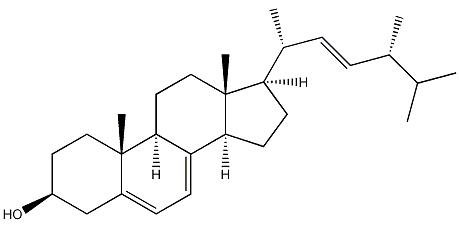
Structural formula
| Business number | 0192 |
|---|---|
| Molecular formula | C28H44O |
| Molecular weight | 396.65 |
| label |
(3?)-Ergot-5,7,22-trienol-3-ol, Ergosterol, 3?-Hydroxy-5,7,22-ergostatriene, 5,7,22-Ergostatrien-3?-ol, Provitamin D2, Ergosterin, (3?,22E)-Ergosta-5,7,22-trien-3-ol, alcohol solvent |
Numbering system
CAS number:57-87-4
MDL number:MFCD00003623
EINECS number:200-352-7
RTECS number:None
BRN number:2338604
PubChem number:24894628
Physical property data
1. Character: white flake or needle crystal. It is easily oxidized to yellow when exposed to sunlight and air.
2. Density (g/mL, 25/4?): 1.04
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 170
5. Boiling point (ºC, normal pressure): 250 (1.33pa)
6. Boiling point (ºC, 1.33Pa): 250
7. Refractive index: Undetermined
8. Flash point (ºC): 216.3
9. Specific rotation (º): [?] D20 -135° (C=1.2, in chloroform)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: 1g of product is soluble in 660ml cold ethanol, 45ml boiling ethanol, 70ml cold ether, 39ml boiling ether, 31ml chloroform, and is almost insoluble in water. Can be precipitated by digitonin.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 124.19
2. Molar volume (cm3/mol): 394.0
3. Isotonic specific volume (90.2K ): 984.4
4. Surface tension (dyne/cm): 38.9
5. Polarizability (10-24cm3): 49.23
CalculateChemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): 7.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
p>
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 20.2
p>
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 712
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 8
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exist in tobacco leaves and smoke.
2. It is the most important phytosterol. When exposed to ultraviolet rays, one of the four carbon rings of the molecule breaks and becomes vitamin D2, which is the raw material for producing vitamin D2.
3. Found in yeast and some plants.
Storage method
This product should be sealed and stored in a cool, dark place at a storage temperature of 4ºC
Synthesis method
1. Extracted from yeast that synthesizes this product from glucose.
2. Tobacco: 1.
Purpose
For biochemical research. It has the effect of vitamin D2.
extended-reading:https://www.cyclohexylamine.net/polyurethane-catalyst-pc41-hard-foam-catalyst-pc41/extended-reading:https://www.bdmaee.net/toyocat-daem-catalyst-tosoh/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/134.jpgextended-reading:https://www.newtopchem.com/archives/90extended-reading:https://www.newtopchem.com/archives/94extended-reading:https://www.cyclohexylamine.net/catalyst-c-225-polyurethane-retardation-catalyst-c-225/extended-reading:https://www.cyclohexylamine.net/nn-dicyclohexylmethylamine-2/extended-reading:https://www.newtopchem.com/archives/44579extended-reading:https://www.bdmaee.net/fomrez-sul-11a-catalyst-momentive/extended-reading:https://www.bdmaee.net/pc-cat-dmp-catalyst-14-dimethylpiperazine-nitro/