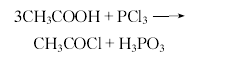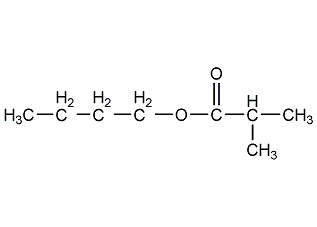
Structural formula
| Business number | 02CV |
|---|---|
| Molecular formula | C8H14O2 |
| Molecular weight | 142.20 |
| label |
n-Butyl isobutyrate, n-butyl methacrylate, butyl methacrylate, Butyl-2-methyl-2-acrylate, n-butyl methacrylate, 2-n-Butyl methacrylate, n-butyl methacrylate, Butyl methacrylate (containing stabilizer hydroquinone), n-Butyl methacrylate, 2-Methyl-2-propenoic acid butyl ester, 2-Methylacrylic acid butyl ester, BMA, paint solvents, petroleum additives, Electronic coating raw materials and intermediates |
Numbering system
CAS number:97-88-1
MDL number:MFCD00009444
EINECS number:202-615-1
RTECS number:OZ3675000
BRN number:773960
PubChem number:24883107
Physical property data
1. Properties: colorless and transparent liquid with sweet and ester smell. [1]
2. Melting point (?): -76.3~-74.9[2]
3. Boiling point ( ?): 160~163[3]
4. Relative density (water=1): 0.90 (20?)[4]
5. Relative vapor density (air=1): 4.8[5]
6. Saturated vapor pressure (kPa): 0.65 (20?)[6]
7. Heat of combustion (KJ/mol): -4891.7[7]
8. Critical pressure (MPa ): 2.6[8]
9. Octanol/water partition coefficient: 2.88[9]
10. Flash Point (?): 41; 54.4 (OC): 52.2 (OC) [10]
11. Ignition temperature: 294[11]
12. Explosion upper limit (%): 8[12]
13. Explosion lower limit (%): 2[13] sup>
14. Solubility: Insoluble in water, miscible in alcohol and ether, and soluble in most organic solvents. [14]
Toxicological data
1. Acute toxicity: Mouse abdominal LD50: 1490 mg/kg; Rabbit transdermal LD50: 11300 mg/kg; Rat oral LD50: 20g/kg; Rat inhalation LC50: 19689mg/m3, 4 hours;
2. It is irritating to the eyes, respiratory system and skin, and may cause allergies in contact with the skin.
3. Acute toxicity[15]
LD50: 16g/kg (rat Oral); 1490mg/kg (mouse intraperitoneal); 11300mg/kg (rabbit transdermal)
LC50: 19689mg/m3 ppm (rat inhalation, 4h)
4. Irritation[16] Rabbit transdermal: 500ul, mild irritation.
5. Subacute and…Sexual toxicity[17] Rat oral administration: 5% LD50, 4 to 6 months (feeding), moderate accumulation.
Ecological data
1. Ecotoxicity[18] EC50: 37~55mg/L (5, 15, 30min) (photobacteria, Microtox Test)
2. Biodegradability[19] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 38% degraded after 28 days.
3. Non-biodegradability[20] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 17h (theoretical).
When the pH value is 11, the hydrolysis half-life is 4 hours.
4. Bioconcentration[21] BCF: 91 (theoretical)
5. Other harmful effects[22] This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 40.45
2. Molar volume (cm3/mol): 158.9
3. Isotonic specific volume (90.2K ): 360.3
4. Surface tension (dyne/cm): 26.4
5. Polarizability: 16.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 127
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is basically the same as methyl methacrylate and has lower toxicity. The oral LD50 in rats is 20mL/kg body weight. The protection requirements are the same as those for methyl methacrylate.
2. Stability[23] Stable
3. Incompatible substances[24] Strong oxidants, strong acids, strong alkali
4. Conditions to avoid contact [25] Heat, light, ultraviolet rays , contact with air
5. Polymerization hazard[26] Polymerization
Storage method
Storage Precautions[27] Usually products contain polymerization inhibitors. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37?. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Methacrylic acid and n-butanol undergo an esterification reaction under the catalysis of sulfuric acid, and then undergo salting out and distillation to obtain the finished product.
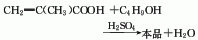
Methacrylic acid and n-butanol The esterification reaction is carried out in the presence of sulfuric acid catalyst and polymerization inhibitor hydroquinone, and then the finished product is obtained through salting out and distillation. See “butyl acrylate”. The reaction formula is as follows: 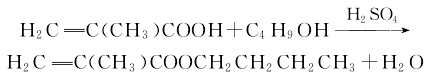
Purpose
1. Organic synthesis, preparation of embedding media for electron microscopy, adhesives for plastics and optical glasses, emulsifiers for textiles, leather shoes and papermaking, solvents for coatings, and petroleum additives.
2. Used as a soft monomer in the manufacture of acrylic solvent-based and emulsion-based adhesives. Its low viscosity polymer can be used as special coatings, paper and leather processing aids, fiber treatment agents, metal surface treatment agents, etc. Poly-n-butyl methacrylate produced from this product is a transparent elastic material and is widely used as the interlayer of aircraft cockpit safety glass and bulletproof glass such as automobiles, as well as precision radio equipment. It can also be copolymerized with the same series of unsaturated esters and acids to produce materials for various uses.
3. Mainly used to manufacture acrylate polymers and copolymers. Used in the manufacture of bulletproof glass and precision radio equipment, and used as oil additives in the petroleum industry.
4. Used in organic synthesis, binders for manufacturing plastics and optical glasses, and auxiliaries for textile, leather and papermaking. [28]
extended-reading:https://www.newtopchem.com/archives/44759extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT4100-catalyst-monobutyl-tin-oxide-FASCAT-4100.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-RP205-Addocat-9727P-high-efficiency-amine-catalyst.pdfextended-reading:https://www.newtopchem.com/archives/43088extended-reading:https://www.newtopchem.com/archives/45094extended-reading:https://www.morpholine.org/dabco-ne1060-non-emissive-polyurethane-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/137-5.jpgextended-reading:https://www.bdmaee.net/dimethylaminoethoxyethanol-cas-1704-62-7-n-dimethylethylaminoglycol/extended-reading:https://www.bdmaee.net/niax-a-1-catalyst-bisdimethylaminoethyl-ether-momentive/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/07/88-1.jpg