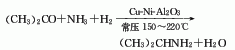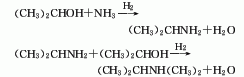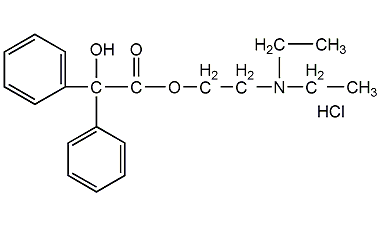
Structural formula
| Business number | 018K |
|---|---|
| Molecular formula | C20H26ClNO3 |
| Molecular weight | 363.88 |
| label |
2-(Diethylamino)ethyl Benzilate Hydrochloride, Benzilic Acid 2-(Diethylamino)ethyl Ester Hydrochloride |
Numbering system
CAS number:57-37-4
MDL number:MFCD00012624
EINECS number:200-324-4
RTECS number:DD2800000
BRN number:None
PubChem number:24891970
Physical property data
1. Appearance: White or almost white crystalline powder. Odorless, slightly bitter taste.
2. Density (g/mL,25/4?)? SPAN>Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):177-181
5. Boiling Point (ºC,Normal pressure): Undetermined
6. Boiling Point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. saturated vapor pressure (kPa,60ºC): Undetermined
13. Burning Heat (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of partition coefficient for water: undetermined
17. Explosion limit (%,V/V): Undetermined
18. Explosion lower limit (%,V/V): Undetermined
19. Solubility:25?100mlThis product can be dissolved in water14.9g, almost insoluble in ether.
Toxicological data
1, acute toxicity: human oral TDLo: 14ug/kg; rat oral LD50: 184mg/kg; rat intraperitoneal LD50: 100mg/kg; mouse oral LD50: .160mg/kg;
Mouse abdominal cavity LD50: 76mg/kg; Mouse subcutaneous LD50: 250mg/kg; Mouse intravenous LD50: 14300ug /kg; mouse intradermal LD50: 350mg/kg; rabbit intraperitoneal LD50?100mg/kg;
Rabbit vein LD50: 15mg/kg; Guinea pig abdominal cavity LD50?100mg/kg
2, reproductive toxicity: male rats subcutaneously TDLo: 500ug/kg, 1 days before mating
Ecological data
None
Molecular structure data
1. Molar refractive index: 94.60
2. Molar volume (m3/mol??293.4
3. isotonic specific volume (90.2K):755.8
4. Surface Tension (dyne/cm):43.9
5. Polarizability(10-24cm3): 37.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 9
5. Number of tautomers: none
6. Topological molecule polar surface area 49.8
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 351
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None
Storage method
This product should be sealed, dry and protected from light.
Synthesis method
Composed of diphenyl glycolic acid and1-Chlorine– 2Diethylaminoethane is obtained by reacting toluene, diphenyl glycolic acid and 1-Chlorine-2-Diethylaminoethane is heated and refluxed in the reaction pot3h, cool and crystallize, and the crude product obtained by filtration is refined and this product is obtained.
Purpose
Anticholinergic drugs.
extended-reading:https://www.newtopchem.com/archives/44080extended-reading:https://www.newtopchem.com/archives/661extended-reading:https://www.newtopchem.com/archives/44928extended-reading:https://www.newtopchem.com/archives/993extended-reading:https://www.bdmaee.net/dabco-mp602-catalyst-cas31506-44-2-evonik-germany/extended-reading:https://www.newtopchem.com/archives/44076extended-reading:https://www.bdmaee.net/dabco-33-s-addocat-106-teda-l33b/extended-reading:https://www.cyclohexylamine.net/trimerization-catalyst-pc-41-triazine-catalyst/extended-reading:https://www.bdmaee.net/dabco-ne210-balance-catalyst-ne210-dabco-amine-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT4102-catalyst-monobutyl-tin-triisooctanoate-CAS-23850-94-4.pdf