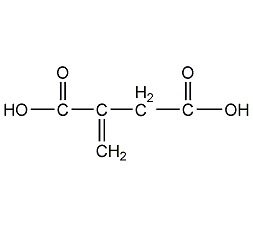
Structural formula
| Business number | 02CJ |
|---|---|
| Molecular formula | C5H6O4 |
| Molecular weight | 130.1 |
| label |
methylene succinic acid, methinesuccinic acid, 2-Propylene-1,2-dicarboxylic acid, methylene succinic acid, Methyl succinic acid, Methylenesuccinicaci, 3-Carboxy-3-butenoic acid, 2-Propene-1,2-dicarboxylic acid, Propylenedicarboxylic acid, sour agent, pH regulator, metal chelating agents, acidic solvent |
Numbering system
CAS number:97-65-4
MDL number:MFCD00004260
EINECS number:202-599-6
RTECS number:None
BRN number:1759501
PubChem number:24881763
Physical property data
1. Properties: white crystalline powder, with special odor and hygroscopicity.
2. Density (g/mL, 20?): 1.632
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 175
5. Boiling point (ºC, normal pressure): 268 (sublimation)
6. Crystal phase standard combustion heat (enthalpy) (kJ ·mol-1): -1983.93
7. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -841.11
8. Flash point (ºC): 198.7
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC ): 800
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13 . Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion Lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in benzene, chloroform, ether, petroleum ether, carbon disulfide, soluble in water, ethanol, and acetone.
Toxicological data
None
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 27.91
2. Molar volume (cm3/mol): 95.2
3. Isotonic specific volume (90.2K ): 259.8
4. Surface tension (dyne/cm): 55.4
5. Polarizability (10-24cm3): 11.06
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -0.1
2. Number of hydrogen bond donors: 2
3. ?Number of bonded receptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular poles Physical surface area (TPSA): 74.6
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 158
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. The number of determined stereocenters of chemical bonds: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
1. Avoid contact with alkalis and oxidants.
2.This product has low toxicity and is not harmful to health, but its vapor is toxic. During production, equipment should be sealed and operators should wear protective gear.
3. The chemical properties are relatively active and can be polymerized by itself or with different numbers of other monomers such as propylene. Polymerization of nitriles, styrene, etc. The vapor is toxic and can easily decompose if overheated.
4. Exist in smoke.
Storage method
Sealed packaging. Store in a cool, dry place away from light. It needs to be put into paper buckets lined with plastic bags and stored tightly. Pay attention to moisture, heat and oxidation protection. Store and transport according to general chemical regulations.
Synthesis method
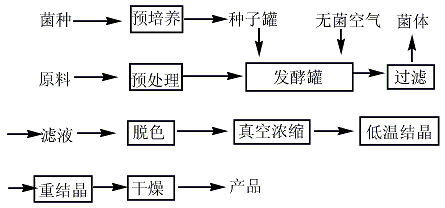
1. There are two types of chemical synthesis methods and industrial fermentation methods. Most manufacturers use industrial fermentation methods for production. The fermentation method mainly uses starch, sucrose, molasses, sawdust, rice straw and other agricultural and sideline products as raw materials, uses sugar as the culture medium, adds nitrogen sources and inorganic salts, and uses Aspergillus terreus as the strain. After two days of fermentation, it is filtered, concentrated, decolorized, and crystallized. , obtained by drying.
2.Heat the concentrated citric acid aqueous solution to 280~300? under reduced pressure (4.0~5.3kPa) to decompose itaconic anhydride and itaconic acid, and then separate and extract itaconic acid from them.
3. Synthesized from propargyl chloride, carbon monoxide, carboxynickel and water.
Purpose
1. Can be used as sour agent, pH regulator, and metal chelating agent. It is an important intermediate in the chemical synthesis industry and can be used to produce synthetic resins, rubber, plastics, lubricant additives, boiler descalers, adhesives, detergents, herbicides, etc. Its esters are also important raw materials for plastic plasticizers.
2.In recent years, more and more research and applications have been conducted on the synthesis of copolymers using itaconic acid as a monomer. One of the important aspects is the use of itaconic acid as a monomer. Conic acid copolymer is used as water treatment agent and chemical cleaning agent. Itaconic acid and acrylic acid or methacrylic acid are polymerized by aqueous solution polymerization to obtain a copolymer with a relative molecular mass of 500 to 200,000. Copolymers with different relative molecular weights and compositions are different properties and uses.
Itaconic acid-acrylic acid copolymer (mass ratio 90:10) is used for online cleaning of circulating cooling water systems, and can effectively remove thick scale layers (0.01~1.7 mm thick). Hard scale containing 67% hydroxyapatite and 13% magnesium silicate adhered to the heating surface. It was treated with 100 mg/L itaconic acid-acrylic acid copolymer (mass ratio 90:10), and the scale removal rate reached 90%. When treated with sodium polyacrylate (average relative molecular weight: 4500), the scale removal rate was only 15.2%. Its descaling characteristics are: it can be carried out while the equipment is running normally, the cleaning conditions are mild, there is no need to add acid to lower the PH value, and the cleaning time is slightly longer (generally 10 to 60 days).
3. As a comonomer of polyacrylonitrile, its synthetic fibers can improve the dyeing properties of synthetic fibers. It can also be used to prepare plasticizers, lubricant additives, dispersion latex for coatings, etc.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/66.jpgextended-reading:https://www.newtopchem.com/archives/40343extended-reading:https://www.newtopchem.com/archives/category/products/page/171extended-reading:https://www.morpholine.org/elastomer-environmental-protection-catalyst-nt-cat-e-129/extended-reading:https://www.bdmaee.net/dabco-rp204-reactive-catalyst-dabco-reactive-catalyst/extended-reading:https://www.cyclohexylamine.net/main-3/extended-reading:https://www.newtopchem.com/archives/664extended-reading:https://www.bdmaee.net/nt-cat-a-1-catalyst-cas3033-62-3-newtopchem/extended-reading:https://www.bdmaee.net/niax-a-577-delayed-gel-type-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/44909