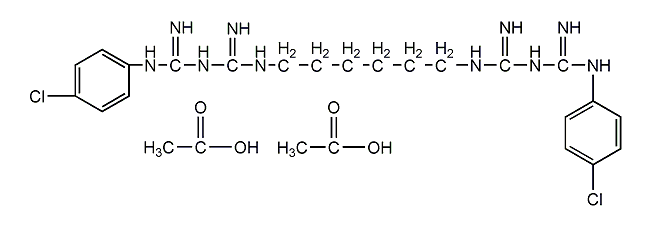
Structural formula
| Business number | 0187 |
|---|---|
| Molecular formula | C22H30Cl2N10·2C2H4O2 |
| Molecular weight | 625.6 |
| label |
1,6-Bis(N5-[p-chlorophenyl]-N1-biguanido)hexane, 1,1?-Hexamethylenebis(5-[p-chlorophenyl]biguanide), Chlorhexidine acetate, Chlorhexidine acetate |
Numbering system
CAS number:56-95-1
MDL number:MFCD00012532
EINECS number:200-302-4
RTECS number:DU1930000
BRN number:None
PubChem number:24892838
Physical property data
1. Characteristics: white crystal sex powder.
2. Density (g/mL,25/4?) ? Undetermined
3. Relative vapor density (g/mL, Air=1): Undetermined
4. Melting point (ºC): 260-262? decomposition
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation ( º): Undetermined
10. Spontaneous ignition point or ignition Combustion temperature (ºC): not OK
11. Vapor Pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure ( kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Oil and water (octanol /Water) partition coefficient pair Value: Undetermined
17. Explosion upper limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): not OK
19.Solubility:20?The solubility in water is1.9g/100ml, soluble in ethanol.Toxicological data
1, Skin or Eye Irritation: Rabbit, Skin Contact, Standard Draize test test, 500mg/24H
2, acute toxicity:; mouse Oral LD50: 2mg/kg; mouse abdominal cavity LD50: 38mg/kg; mouse subcutaneous LD50: 325mg/kg; mouse intravenous LD50: 25mg/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 8
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 13
5. Number of tautomers: 36
6. Topological molecular polar surface area 252
7. Number of heavy atoms: 42
8. Surface charge: 0
9. Complexity: 680
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12.The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 2
14. The uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 3
Properties and stability
None yet
Storage method
This product should be sealed and stored in a dry and dark place.
Synthesis method
It is prepared from p-chloroaniline through diazotization, condensation, elimination of denitrification, condensation, and neutralization to form a salt.
Purpose
Disinfectants. It has a strong bactericidal effect on Gram-positive bacteria, negative bacteria and fungi, and is also effective on Pseudomonas aeruginosa. Used for skin disinfection, wound irrigation, and surgical instrument disinfection.
extended-reading:https://www.cyclohexylamine.net/dabco-ne1070-gel-type-low-odor-catalyst/extended-reading:https://www.morpholine.org/category/morpholine/dimethomorph/extended-reading:https://www.newtopchem.com/archives/44159extended-reading:https://www.newtopchem.com/archives/45010extended-reading:https://www.bdmaee.net/cas-3855-32-1/extended-reading:https://www.cyclohexylamine.net/category/product/page/13/extended-reading:https://www.bdmaee.net/jeffcat-td-33a-catalyst-cas107-16-9-huntsman/extended-reading:https://www.bdmaee.net/pc-cat-np70-catalyst-nn-dimethylethylaminoethylene-glycol/extended-reading:https://www.bdmaee.net/nt-cat-pt1003/extended-reading:https://www.bdmaee.net/pc5-catalyst/