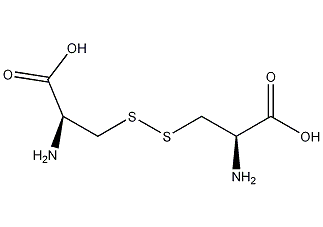
Structural formula
| Business number | 0183 |
|---|---|
| Molecular formula | C6H12N2O4S2 |
| Molecular weight | 240.3 |
| label |
(R,R)-3,3′-dithiobis(2-aminopropionic acid), Dithioaminopropionic acid, Bis-beta-thioalanine, disulfidealanine, (R,R)-3,3′-Dithiobis(2-aminopropionicacid), Cystine, L-Dicysteine, (R,R)-(-)Cystine, L-cystinic acid, biochemical reagents, intermediates, amino acids |
Numbering system
CAS number:56-89-3
MDL number:MFCD00064228
EINECS number:200-296-3
RTECS number:HA2690000
BRN number:1728094
PubChem number:24893101
Physical property data
1. Properties: White hexagonal plate-shaped crystals or crystalline powder. Odorless and tasteless.
2. Density (g/mL, 25/4?): 1.677
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): point 260~261? (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º): [?]D20 -223.4° (C=1, in 1mol/L hydrochloric acid).
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% ,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: Soluble in dilute acid and alkaline solutions, Very slightly soluble in water, insoluble in ethanol, ether, benzene and chloroform.
Toxicological data
1. Acute toxicity: Rat oral LD50: 25mg/kg2.Other multiple dose toxicity: rat oral TDLo: 279mg/kg/93D-C
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 55.95
2. Molar volume (cm3/mol): 152.8
3. Isotonic specific volume (90.2K): 468.6
4. Surface tension (dyne/cm): 88.2
5. Polarizability (10-24cm3): 22.18
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -6.3
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 8
p>
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 177
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 192
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 2
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable properties under normal temperature and pressure.
2. Found in tobacco leaves.
3. Widely found in hair, hair, bones and horns.
4. There are three isomers: left-handed body, right-handed body and racemate.
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
1. L-cystine was discovered in 1810 by Wollaston from bladder stones. In 1832, Berzelius named it cystine. It is a sulfur-containing amino acid that exists in small amounts in proteins, mostly in the keratin of hair, fingers and claws. It can also be obtained synthetically. Industrially extracted from hair, the yield can reach 7.5-8%. In actual production, some only reach 5%.
2.Hydrolyze pig hair in hydrochloric acid, filter to remove impurities, crystallize to obtain crude product, add activated carbon for decolorization and decolorizationPrepared from iron, washing and drying.
3.
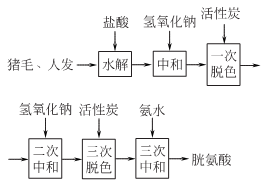
Add 720kg of hydrochloric acid with a concentration of 10mol/L into the hydrolysis tank and heat it to 70~80?. Quickly add 400kg of human hair or pig, continue to heat to 100?, and raise the temperature to 110~117? within 1~1.5h, hydrolyze for 6.5~7h (from 100?), cool and filter. Add 30% to 40% industrial sodium hydroxide solution to the filtrate under stirring. When the pH value reaches 3.0, add alkali solution at a slow speed until the pH value is 4.8. Leave it for 36 hours, separate the precipitate, and centrifuge to dryness to obtain bladder. Crude acid product (I), the mother liquor contains glutamic acid, arginine and leucine, etc. Weigh 150kg of crude cystine (I), add about 90kg of 10mol/L hydrochloric acid and 360kg of water, heat to 65~70°C, stir and dissolve for 0.5h, then add 12kg of activated carbon, heat to 80~90°C, and keep warm for 0.5h. Plate and frame filter press. Heat the filtrate to 80-85°C, add 30% sodium hydroxide while stirring, and stop when the pH reaches 4.8. Let it stand for the crystals to precipitate, siphon the supernatant, separate the bottom precipitate, and then centrifuge and spin dry to obtain crude cystine (II). Weigh 100kg of crude cystine (II), add 500L of 1mol/L hydrochloric acid, heat to 70°C, and then add 3 to 5kg of activated carbon. Then the temperature was raised to 85°C, kept stirred for 0.5h, and plate and frame filtered. Add distilled water about 1.5 times the volume of the filtrate to the filtrate, heat it to 75-80°C, and neutralize it with 12% ammonia water to pH 3.5-4.0 while stirring. At this time, cystine crystals will precipitate. The crystals are centrifuged to dryness, washed with distilled water until there is no chloride ion, and vacuum dried to obtain the finished product of cystine. The yield of human hair can reach 8%, and the yield of pig hair can reach 5%.
Purpose
1. Used for biochemical research. Preparation of biological culture media. It is used in biochemistry and nutrition research. In medicine, it can promote the oxidation and reduction functions of body cells, increase white blood cells and prevent the development of pathogenic bacteria. Mainly used for various types of alopecia. It is also used for acute infectious diseases such as dysentery, typhoid, and influenza, asthma, neuralgia, eczema, and various poisoning diseases, and has the effect of maintaining protein configuration. Also used as food flavoring.
2.Biochemical reagents, used for the preparation of biological culture media. It is also an important component of amino acid infusion and complex amino acid preparations.
3.Used as feed nutritional fortifier, it is beneficial to animal development, increases body weight and liver and kidney function, and improves fur quality.
4.Can be used as a cosmetic additive, which can promote wound healing, prevent skin allergies and treat eczema.
extended-reading:https://www.bdmaee.net/dabco-xd-104-dabco-tertiary-amine-catalyst-catalyst-xd-104/extended-reading:https://www.bdmaee.net/dabco-t-96-catalyst-cas103-83-3-evonik-germany/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-NE1060-catalyst–NE1060-foam-catalyst–NE1060.pdfextended-reading:https://www.cyclohexylamine.net/polyurethane-tertiary-amine-catalyst-catalyst-r-8020/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/07/90-1.jpgextended-reading:https://www.bdmaee.net/fascat2004-catalyst-cas7772-99-8-stannous-chloride/extended-reading:https://www.newtopchem.com/archives/999extended-reading:https://www.newtopchem.com/archives/40462extended-reading:https://www.newtopchem.com/archives/39838extended-reading:https://www.newtopchem.com/archives/39778