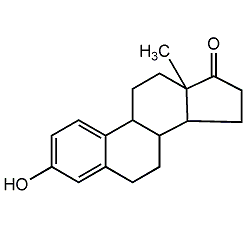
Structural formula
| Business number | 015W |
|---|---|
| Molecular formula | C18H22O2 |
| Molecular weight | 270.37 |
| label |
estrogen, Estrone; estrone; 3-hydroxyestradiol 1,3,5-(10)-trien-17one; estrogen; oxysterol; estradiol; folliculin;, 1,3,5(10)-Estratrien-3-ol-17-one, 3-Hydroxy-1,3,5(10)-estratrien-17-one, Folliculin, Theelin, Kolpon, Ketohydrocyestrin, Oestrone, for uterine agenesis |
Numbering system
CAS number:53-16-7
MDL number:MFCD00003620
EINECS number:200-164-5
RTECS number:KG8575000
BRN number:1915077
PubChem number:24278427
Physical property data
1. Properties: White to milky white crystalline powder
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g /mL, air=1): Undetermined
4. Melting point (ºC): 280
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º): +158°?+165°
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure ( kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient relationship Value: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Almost insoluble in water. Soluble in ethanol, chloroform, boiling ethanol, acetone, dioxane and vegetable oil. Slightly soluble in absolute ethanol, ether and alkali.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 78.04
2. Molar volume (cm3/mol): 232.1
3. Isotonic specific volume (90.2K ): 604.7
4. Surface tension (dyne/cm): 46.0
5. Polarizability (10-24cm3): 30.94
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 18
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 418
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 4
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Estrone is an estrogen secreted by mature ovarian follicles (mainly theca cells) and the corpus luteum. It has the same effect as estradiol and can promote and regulate female accessory sexual organs. development, prompting the emergence of female secondary sexual characteristics.
Storage method
Store in a sealed and protected place from light
Synthesis method
1. Made from 17?-estradiol under the action of 17?-hydroxysteroid dehydrogenase.
2.
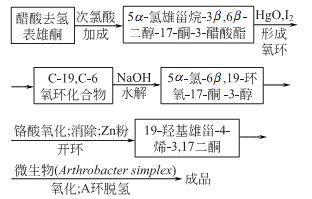
Purpose
1. It is mainly used to treat uterine hypoplasia, menstrual disorders, menopausal disorders, etc. It is rarely used due to its difficult source.
extended-reading:https://www.newtopchem.com/archives/40439extended-reading:https://www.newtopchem.com/archives/category/products/page/51extended-reading:https://www.bdmaee.net/butyltin-tris2-ethylhexanoate-2/extended-reading:https://www.cyclohexylamine.net/high-quality-nn-dicyclohexylmethylamine-cas-7560-83-0/extended-reading:https://www.bdmaee.net/jeffcat-dmea-catalyst-cas107-15-3-huntsman/extended-reading:https://www.bdmaee.net/butyl-tin-triisooctoate-cas23850-94-4-fascat9102-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/126extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/138-2.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/123extended-reading:https://www.newtopchem.com/archives/44882