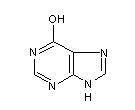
Structural formula
| Business number | 01FE |
|---|---|
| Molecular formula | C5H4N4O |
| Molecular weight | 136.11 |
| label |
1,7-dihydro-6H-purin-6-one, 6-Hydroxypurine, 6-Hydroxypurine, 6-oxopurine, Hypoxanthine, 6-hypoxanthine, Purine-6(1H)-one |
Numbering system
CAS number:68-94-0
MDL number:MFCD00005725
EINECS number:200-697-3
RTECS number:UP0791000
BRN number:5811
PubChem number:24895850
Physical property data
1. Character:Needle crystal
2. Density (g/mL,25/4?): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):>360
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. saturated vapor Pressure (kPa,60ºC ): Unsure
13. Heat of combustion (KJ/mol): Unsure
14. Critical temperature (ºC): Unsure
15. Critical pressure (KPa): Unsure
16. Oil and water (octanol/Log value of water) partition coefficient: Uncertain
17. Explosion limit ( %,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:Almost insoluble in water0.078/100m1(19?)1.4g/100ml(100?) . Soluble in dilute acids and alkalis, such as 0.5mol/LSulfuric acid or10mol /LSodium hydroxide
Toxicological data
Acute toxicity: mouse abdominal D50: 750 mg/kg;
Reproduction: mouse abdominal cavityTDLo?600 mg/kgSEX/DURATION : female 13 day(s) after conception;
Mouse abdominal cavityTDLo?1 gm/kgSEX/DURATION : female 13 day(s) after conception;
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 8
6. Topological molecule polar surface area 70.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 190
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Together with xanthine, it is widely present in animals and plants. The inosinic acid of its nucleotide is the precursor of the purine nucleotide of nucleic acid. It is also the parent body of caffeine. Hypoxanthine can be produced through the action of adenine deaminase or nitrite.Purine deamination can also result in the phosphorolysis of inosine through nucleoside phosphorylase.
Storage method
This product should be kept sealed, cool and dry.
Synthesis method
Ethyl cyanoacetate, sodium ethoxide, and thiourea The combination reaction gives 2- Thiol-4-Amino -6-Hydroxypyrimidine , and then undergo nitrosation, reduction, elimination, and cyclization to obtain 6-Hydroxypurine.
Purpose
Anti-malignant tumor drugs6-Intermediate of mercaptopurine.
extended-reading:https://www.cyclohexylamine.net/low-odor-catalyst-9727-reaction-type-catalyst-9727/extended-reading:https://www.cyclohexylamine.net/polyurethane-monosodium-glutamate-self-skinning-pinhole-elimination-agent/extended-reading:https://www.cyclohexylamine.net/di-n-octyltin-oxide-dioctyltin-oxide-xie/extended-reading:https://www.bdmaee.net/cas7560-83-0/extended-reading:https://www.cyclohexylamine.net/c-225-foaming-retarder-c-225/extended-reading:https://www.bdmaee.net/polyurethane-rigid-foam/extended-reading:https://www.bdmaee.net/polyurethane-amine-catalyst-9727/extended-reading:https://www.newtopchem.com/archives/category/products/page/129extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-DC1-delayed-catalyst–DC1-delayed-strong-gel-catalyst–DC1.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/10.jpg