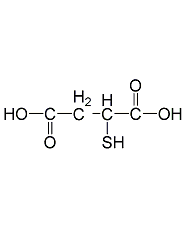
Structural formula
| Business number | 01G5 |
|---|---|
| Molecular formula | C4H6O4S |
| Molecular weight | 150.15 |
| label |
Thiomalic acid, thiomalic acid, Mercaptosuccinic acid, Thiosuccinic acid, Thiomalic acid, HOOCCH(SH)CH2COOH |
Numbering system
CAS number:70-49-5
MDL number:MFCD00004860
EINECS number:200-736-4
RTECS number:WM8225000
BRN number:1099858
PubChem number:24889100
Physical property data
1. Properties: colorless crystals or white powder. There is an odor of sulfide
2. Density (g/mL, 25/4?): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 149-1505. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7 . Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain Determine
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa ): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Easily soluble in water, alcohol, ether and acetone, almost insoluble in benzene
Toxicological data
Acute toxicity: mouse abdominal LD50: 200 mg/kg;
Ecological data
None
Molecular structure data
1. Molar refractive index: 31.56
2. Molar volume (cm3/mol): 98.2
3. Isotonic specific volume (90.2K ): 284.1
4. Surface tension (dyne/cm): 69.9
5. Polarizability (10-24cm3): 12.51
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.4
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 75.6
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the atomic stereocenter Quantity: 0
12. Uncertain number of atomic stereocenters: 1
13. Determined number of chemical bond stereocenters: 0
14. Uncertain chemical bonds Number of stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed, cool, dry and protected from light.
Synthesis method
Hydration of maleic anhydride produces maleic acid, which is then reacted with hydrogen sulfide to produce this product. For laboratory preparation, 23.2g malic acid and 20g thioacetic acid can be dissolved in 80ml ethyl acetate and refluxed for 1 hour. After cooling, add 400 ml of benzene to extract, add activated carbon for decolorization, filter to remove the carbon, and recover benzene to obtain crude product. After purification, 32g of acetylthiomalic acid was obtained, with a melting point of 126°C. Further hydrolysis yields mercaptosuccinic acid.
Purpose
This product is the main component of cold perm and is also used in complex concealment, biochemical research, heavy metal detoxifier and rubber industry.
extended-reading:https://www.newtopchem.com/archives/1029extended-reading:https://www.morpholine.org/n-methylimidazole/extended-reading:https://www.bdmaee.net/cas-27253-29-8/extended-reading:https://www.newtopchem.com/archives/1095extended-reading:https://www.bdmaee.net/niax-sa-800-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/nt-cat-k2097-catalyst-cas127-08-2-newtopchem/extended-reading:https://www.newtopchem.com/archives/805extended-reading:https://www.newtopchem.com/archives/39978extended-reading:https://www.bdmaee.net/n-methylmorpholine/extended-reading:https://www.cyclohexylamine.net/category/product/page/19/