Gamma-Butyrolactone GBL CAS96-48-0 Gamma-Martinolactone
Overview:
Chinese name: ?-butyrolactone (GBL)
1,4-Butyrolactone, also known as ?-butyrolactone, is an organic compound, chemical formula C4H6O2, molecular weight 86.089, is a colorless transparent liquid, used in the production of cyclopropylamine, pyrrolidone and other drugs, but also used as a solvent, diluent, curing agent, etc.
Synonyms: gamma-butyrolactone
Gamma-butyrolactone
1,4-Butyrolactone
?-Hydroxybutyric acid lactone
Gamma-butyrolactone (gamma-butyrolactone)
English name: gamma-butyrolactone
Molecular formula(Formula): C4H6O2
Molecular Weight(Molecular Weight): 120.104
CAS No.: 96-48-0
Chinese name: gamma-butyrolactone, gamma-butyrolactone, 1,4-butyrolactone, gamma-hydroxybutyrate, gamma-butyrolactone (gamma-butyrolactone)
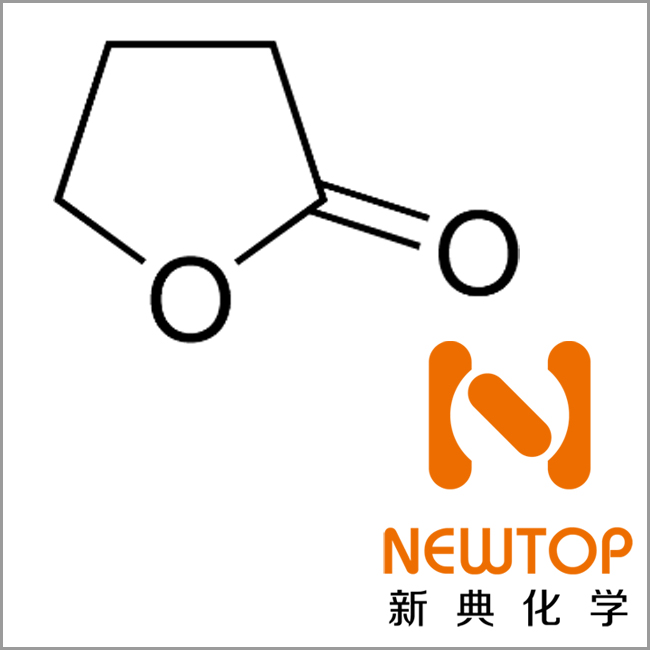
Molecular structure: See figure
Molecular formula: C4H6O2
Molecular weight: 120.104
CAS No.: 96-48-0
EINECS No.: 202-509-5
Molecular weight 120.104
Physical and chemical properties
Appearance Colorless transparent liquid.
Boiling point 35?
Freezing point
Relative density 1.129
Refractive index 1.4348
Flash point 98?
Solubility Miscible with water and general organic solvents. Slightly soluble in aliphatic hydrocarbons.
Uses:
1, ?-butyrolactone is a kind of high boiling point solvent with strong solubility, safe and convenient to use and manage, used as extraction agent of butadiene, aromatic hydrocarbons and advanced grease in petroleum processing;
2?It is used as the spinning solvent of acrylonitrile fiber in chemical fiber industry, and is the dyeing auxiliary of wool, nylon, acrylonitrile and other fibers.
3?In addition, it is also widely used in lithium-ion battery electrolyte and super energy storage capacitor.
Applications:
Used in the production of cyclopropylamine, pyrrolidone and other drugs, but also industrial solvents, diluents, curing agents, etc.
Stability:
- Avoid contact with strong oxidizing agents, strong acids, strong bases, strong reducing agents.
Soluble in water, methanol, etc., non-corrosive to metal, flammable, low toxicity, easily absorbed by skin, should be prevented from contact with skin.
Chemical properties: It is more stable compound. Easily hydrolyzed under the action of hot alkali, hydrolysis is reversible, when pH = 7, and then generate lactone. Hydrolysis is slower in acidic medium.
- It is a low toxicity substance, with anesthetic effect on the central nervous system, oral LD50=1800mg/kg in rats, irritating to the skin, and its smoke has irritating effect on eyes, mucous membrane and upper respiratory tract.
- present in roasted tobacco, white rib tobacco, spice tobacco and smoke.
- Present in apricot, bread, coffee, also volatile aromatic component of fried hazelnut.
- Method of preparation:
Add 2mL of dry acetonitrile and 110mg of trimethylchlorosilane (1.0mmol) to the reaction flask, add 187g of silver nitrate (1.1mmol) at 0? under nitrogen protection and stir the reaction for 1h. Pour out the supernatant (remove the silver chloride) and add to CrO3150mg (1.5mmol) with stirring.
(1.5 mmol) in 1 mL acetonitrile and stirred for 15 min to obtain TMSONO2- CrO3 oxidizer. The solution of THF (2) (1.0 mmol) dissolved in 1 mL acetonitrile was added slowly dropwise under the cooling of ice water bath, and the reaction was stirred at room temperature for 24 h. The product was filtered, washed with dichloromethane, and the solvent was evaporated to obtain the crude product ? (1). Purified by silica gel column in 65% yield.
[ Solubility in water ]: MISCIBLE
[ Molecular structure ]:
1? Molar refractive index: 20.18
2? Molar volume (cm³/mol): 76.2
3? Isotropic specific volume (90.2K): 186.0
4? Surface tension (dyne/cm): 35.4
5? Dielectric constant:
6? Dipole distance (10-²?cm³):
7, Polarization rate: 8.00
[ Calculated Chemistry ]:
- Reference value for calculation of hydrophobic parameters (XlogP):None
- Number of hydrogen bond donors:0
- Number of hydrogen bond acceptors:2
- Number of rotatable bonds:0
- Number of reciprocal isomers:2
6.Topological molecule polar surface area 26.3
7.Number of heavy atoms:6
- Surface charge:0
9.Complexity:67.9
10.Number of isotope atoms:0
11.Number of determined atomic structure centers:0
12.Number of uncertain atomic structure centers:0
13.Number of definite bonding centers:0
14.Number of indeterminate bonding centers:0
15.Number of covalent bonding units:1
[ more ]:
- properties:colorless to yellow oily liquid with cream-like aromatic aroma.
- Relative density (15?): 1.1286
- relative vapor density (g/mL,air=1): 3.0
- melting point (?): -44
- boiling point (?, atmospheric pressure): 206
- Viscosity (mPa- s,25?): 1.7
- refractive index (26.5?): 1.4343
- Flash point (?): 99.2
- Self-ignition point or ignition temperature (?): 455
- Vapor pressure (mmHg,20?): 1.5
- saturation vapor pressure (kPa, 20 ?): 2.0
- heat of evaporation (KJ/mol,204?): 52.3
- critical temperature (?): 457.85
- critical pressure (KPa): 5.131
- specific heat capacity (KJ/(kg- K),25?,constant pressure): 1.67
- specific heat capacity (KJ/(kg- K), 60 ?, constant pressure): 1.88
- upper explosion limit (%, V/V): 16
- lower explosion limit (%,V/V): 1.4
- solubility: miscible with water, also soluble in methanol, ethanol, ether and benzene and other organic solvents. 20.
- relative density (20?, 4?): 1.1299
- Refractive index at room temperature (n20): 1.4346
- Refractive index at room temperature (n25): 1.4348
- solubility parameter (J- cm-3) 0.5: 25.593
- van der Waals area (cm²?- mol-1): 6.250× 109
- van der Waals volume (cm³- mol-1): 45.890
- liquid phase standard thermal fusion (J- mol-1- K-1): 140.0
Storage and transportation:
Should be kept sealed and stored in a dry, cool and ventilated warehouse
Package:
200KG/drum Storage: It is recommended to store in dry and cool area with proper ventilation. Please fasten the lid as soon as possible after the original packaging to prevent the mixing of other substances such as water and other substances from affecting the product performance. Do not inhale dust and avoid skin and mucous membrane contact. Smoking, eating and drinking are prohibited in the workplace. After work, shower and change clothes. Store contaminated clothes separately and wash them before use. Maintain good hygiene habits.