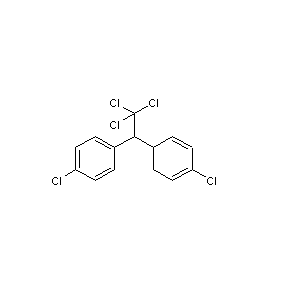
Structural formula
| Business number | 013F |
|---|---|
| Molecular formula | C14H9Cl5 |
| Molecular weight | 354.49 |
| label |
4,4′-DDT, DDT powder, 1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane, 2,2-bis(p-chlorophenyl)-1,1,1-trichloroethane, 1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane, 1,1-bis(4-chlorophenyl)2,2,2-trichloroethane, 4,4′-DDT, Agritan, Azotox, Bovidermol, Clofenotan, Deoval, Detox, Detoxan, Dibovin, Dicophane, Dodat, Organochlorine pesticides |
Numbering system
CAS number:50-29-3
MDL number:MFCD00000802
EINECS number:200-024-3
RTECS number:KJ3325000
BRN number:1882657
PubChem number:24864083
Physical property data
1. Properties: The pure product is white crystal, and the industrial product is white granular or light yellow block containing oil
2. Density (g/mL, 25/4?): 1.55
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 107-110 °C (lit.)
5. Boiling point (ºC, normal pressure): 260?
6. Boiling point (ºC, 5.2 kPa): Undetermined
7. Refractive index: Undetermined
p>
8. Flash point (ºC): 72 °C
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature ( ºC): Undetermined
11. Vapor pressure (kPa, 25 ºC): Undetermined
12. Saturated vapor pressure (kPa, 60 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined 19. Solubility: Easily soluble in pyridine and dioxane. ?The solubilities in 100ml of solvent are: acetone 58g, carbon tetrachloride 45g, chlorobenzene 74g, ethanol 2g, and ether 28g. Insoluble in water, dilute acids and alkalis
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 84.50
2. Molar volume (cm3/mol): 244.1
3. Isotonic specific volume (90.2K ): 638.9
4. Surface tension (dyne/cm): 46.8
5. Polarizability (10-24cm3): 33.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 250
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
50% gel suspension, 2kg plastic bottle, plus carton, net weight 20kg per box. Contact with alkaline substances should be avoided, stored in a cool place, and the packaging equipment should be sealed to prevent fire.
Synthesis method
It is derived from the condensation of trichloroacetaldehyde and monochlorobenzene in the presence of fuming sulfuric acid. Put trichloroacetaldehyde and chlorobenzene into the condensation pot, and add fuming sulfuric acid dropwise while stirring. The condensed material is allowed to stand for stratification, and the waste acid is discharged. The acidic DDT-chlorobenzene solution is washed with hot water and then with sodium hydroxide solution. Then the chlorobenzene is recovered by distillation, and the residue, namely molten DDT, is placed in a drum crystallizer for cooling and crystallization to obtain the finished product. The condensation reaction is carried out at 10-23°C. During the condensation, the waste acid produced by the condensation reaction (including p-chlorobenzenesulfonic acid generated by the side reaction) is added at the same time, or the side reaction is suppressed to reduce the amount of p-chlorobenzenesulfonic acid generated, and sulfuric acid is added dropwise. The time is about 2.5h. The para-position content of first-grade DDT raw powder is ?74.0%.
Purpose
DDT was once one of the widely used pesticides. It has stomach poisoning and contact effects, and can be processed into powder, emulsion or oil. In our country, it was mainly used to control cotton bud and boll-stage pests, fruit tree borers, field crop armyworms, vegetable cabbage caterpillars, etc. It is also used for environmental sanitation and prevention of mosquitoes, flies, bed bugs, etc. DDT is not easily degraded into non-toxic substances, and it can easily accumulate during use and pollute the environment. DDT remaining in plants can enter the bodies of humans and animals through the “food chain” or other ways, causing poisoning and affecting human health. Its use is currently prohibited. However, some industrial uses of DDT, including pesticides using it as raw material, also require DDT as an intermediate, such as dicofol.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/27.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/139-1.jpgextended-reading:https://www.bdmaee.net/c6h11no2/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/3-9.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2019/10/1-2-1.jpgextended-reading:https://www.bdmaee.net/butyltin-mercaptide-2/extended-reading:https://www.newtopchem.com/archives/40466extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-NE1060-catalyst–NE1060-foam-catalyst–NE1060.pdfextended-reading:https://www.bdmaee.net/jeffcat-zf-10-catalyst-cas83016-70-0-huntsman/extended-reading:https://www.newtopchem.com/archives/1776

