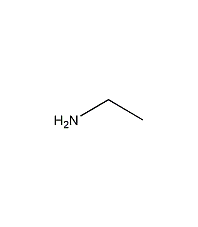
Structural formula
| Business number | 01HV |
|---|---|
| Molecular formula | C2H7N |
| Molecular weight | 45 |
| label |
1-aminoethane, Aminoethane, Ethanamine, 1-Aminoethane, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:75-04-7
MDL number:MFCD00008160
EINECS number:200-834-7
RTECS number:KH2100000
BRN number:505933
PubChem ID:None
Physical property data
1. Properties: colorless liquid or gas with strong ammonia smell. [1]
2. Melting point (?): -81[2]
3. Boiling point (?): 16.6[3]
4. Relative density (water = 1): 0.70[4]
5. Relative vapor Density (air=1): 1.56[5]
6. Saturated vapor pressure (kPa): 121 (20?)[6]
7. Heat of combustion (kJ/mol): -1713.3[7]
8. Critical temperature (?): 182.9[8]
9. Critical pressure (MPa): 5.62[9]
10. Octanol/water partition coefficient: -0.13[10]
11. Flash point (?): -17 (CC); <-6.7 (OC) [11]
12. Ignition temperature (?): 385[12]
13. Explosion upper limit (%): 14.0[13]
14. Lower explosion limit (%): 3.5[14]
15. Solubility: soluble in water, ethanol, ether, etc. [15]
Toxicological data
1. Acute toxicity[16] LD50: 400mg/kg (rat oral); 390mg/kg (rabbit dermal)
2. Irritation[17]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 250?g (24h), or 5mg, severe irritation.
3. Subacute and chronic toxicity [18] Rabbit inhaled 184mg/m3, 7 hours each time, Five times a week, a large number of lungs, peribronchitis and renal parenchyma degeneration were seen in 6 weeks. Starting from 2 weeks after exposure, rabbit eyes showed epithelial cell erosion and corneal edema.
4. Others[19] LCLo: 3000ppm (rat inhalation, 4h)
Ecological data
1. Ecotoxicity[20]
EC50: 40mg/L (48h) (Daphnia)
IC50: 1.3~2.3mg/L (72h) (algae)
2. Biodegradability[21] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 59%~90% degradation after 2 weeks.
3. Non-biodegradability[22] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 14h (theoretical).
Molecular structure data
1. Molar refractive index: 14.84
2. Molar volume (cm3/mol): 65.2
3. Isotonic specific volume (90.2K ): 140.7
4. Surface tension(dyne/cm): 21.5
5. Polarizability (10-24cm3): 5.88
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26
p>
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: The aqueous solution is alkaline, and its chemical properties are similar to those of methylamine. It is unstable to light and will decompose to produce hydrogen, chlorine, ammonia, methane, ethane, etc. when exposed to ultraviolet light at 140~200°C. Pyrolysis occurs at 490~555? under low pressure to generate hydrogen, chlorine, methane, etc. Ethylamine reacts with sodium hypochlorite to form N-chloroethylamine. Chlorine gas is passed into the ethylamine aqueous solution to generate N,N-dichloroethylamine, which reacts with metal sodium and strontium to generate metal ethylamino compounds.
2. Stability[23] Stable
3. Incompatible substances[24] Strong oxidizing agent, strong acid
4. Polymerization hazard[25] No polymerization
5. Decomposition products[26] Ammonia
Storage method
Storage Precautions[27] Stored in a cool, ventilated warehouse dedicated to flammable gases. Keep away from fire and heat sources. The storage temperature should not exceed 30?. Keep container tightly sealed. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with leakage emergency response equipment.
Synthesis method
1. Ethanol (gas phase) ammoniation method: Add ethanol and ammonia in a ratio of 4:1, at a reaction temperature of 350-400°C, a pressure of 2.45-2.94MPa, and use alumina as a dehydration catalyst. In addition to generating ethylamine, side reactions generate diethylamine, triethylamine, ether, acetonitrile and ethylene. After the crude product obtained by condensing the reaction gas is steamed with ammonia, ethylamine is steamed out with a content of more than 95%. Generally, 50% aqueous solution is used as a commodity. Each ton of 50% aqueous solution consumes about 1,400kg of ethanol (95%).
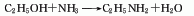
2. Acetaldehyde, hydrogen ammonia The chemical method uses acetaldehyde, hydrogen and ammonia as raw materials and nickel as a catalyst to react to produce ethylamine. First, a structural catalyst with nickel as the main catalyst, reduced copper and reduced chromium as the cocatalyst and kaolin as the carrier is loaded into the reactor, and acetaldehyde is introduced into the reactor at an air velocity of 0.03-0.15L·h-1 to allow hydrogen and acetaldehyde to mix. The ratio is 5:1, and the ratio of ammonia and acetaldehyde is 0.4-3:1. The feedstock is vaporized and entered into the reactor at 80°C. The reaction temperature is controlled between 105-200°C. The product is cooled at -5°C and mono-, di- and triethylamine are separated. Since monoethylamine is formed faster than diethylamine and triethylamine, it is easier to obtain a large amount of ethylamine. When the main product is required to be di- and triethylamine, the generated monoethylamine needs to be recycled into the reaction system for reaction.
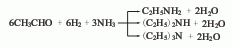
Refining method: Ethylamine is often contained Amines such as diethylamine and triethylamine, as well as impurities such as acetaldehyde, a synthetic raw material. It is fractionated and refined under nitrogen flow, then dehydrated with solid potassium hydroxide, decarbonated and dried with sodium fluorenone for later use. If ethylamine hydrochloride is refined, it can be recrystallized with absolute ethanol or a mixture of methanol and chloroform. The melting point is 109~110°C.
Purpose
1. Used in the production of pesticide triazine herbicides, including atrazine and simazine ([122-34-9]). Both herbicides use cyanuric chloride as raw materials and have similar production processes. Atrazine (also known as Trazine) has a wider application range than simazine and can kill simazine-resistant weeds. It was developed and produced by the Swiss company Geigy in 1985 and later developed into the world’s largest-produced herbicide. Ethylamine is also used in the production of dyes, rubber accelerators, surfactants, antioxidants, ion exchange resins, aircraft fuels, solvents, detergents, lubricants, metallurgical dressing agents, as well as cosmetics and pharmaceuticals.
2. Used in dye synthesis and as extraction agents, emulsifiers, pharmaceutical raw materials, reagents, etc. [28]
extended-reading:https://www.cyclohexylamine.net/category/product/page/9/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/38-7.jpgextended-reading:https://www.cyclohexylamine.net/category/product/page/10/extended-reading:https://www.newtopchem.com/archives/45097extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-15.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-NE1070-polyurethane-gel-type-catalyst–low-odor-catalyst.pdfextended-reading:https://www.bdmaee.net/dabco-xd-102-catalyst-cas106317-60-3-evonik-germany/extended-reading:https://www.bdmaee.net/sponge-foaming-catalyst-smp/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dioctyl-dimaleate-di-n-octyl-tin-CAS33568-99-9-Dioctyl-dimaleate-di-n-octyl-tin.pdfextended-reading:https://www.bdmaee.net/niax-a-337-delayed-tertiary-amine-catalyst-momentive-2/

