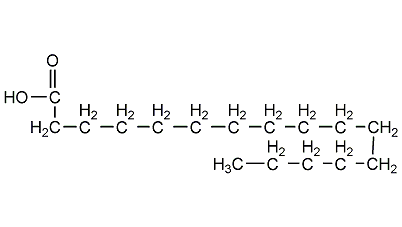
Structural formula
| Business number | 018C |
|---|---|
| Molecular formula | C16H32O2 |
| Molecular weight | 256.43 |
| label |
Hexadecanoic acid, Palmitic acid, cetacetic acid, n-Hexadecanoic acid, Hexadecylic acid, Cetylic acid, Lubricants, softeners, acidic solvent |
Numbering system
CAS number:57-10-3
MDL number:MFCD00002747
EINECS number:200-312-9
RTECS number:RT4550000
BRN number:607489
PubChem number:24898674
Physical property data
1. Characteristics: White, pearlescent scales.
2. Density (g/mL, 25/4?): 0.8527
3. Relative density (25?, 4?): 0.841480
4. Melting point (ºC): 63
5. Boiling point (ºC, normal pressure): 351,271.5 (13.3kpa)
6. Boiling point (ºC, normal pressure): 351,271.5 (13.3kpa)
6. ºC, 13.3kPa): 267
7. Refractive index (n60D): 1.43345
8. Refractive index at room temperature (n20): 1.430970
9. Refractive index at room temperature (n25): 1.427280
10. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -10132.4
11. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -737.2
12. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -10031.1
13. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -838.5
14. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1): -9977.96
15. Crystalline phase standard claimed heat (enthalpy) (kJ ·mol-1): -891.48
16. Crystal phase standard entropy (J·mol-1·K-1): 452.37
17. Crystal phase standard formation free energy (kJ·mol-1): -315.10
18. Explosion lower limit (% ,V/V): Undetermined
19. Solubility: Insoluble in water, slightly soluble in cold alcohol and petroleum ether, easily soluble when heated, soluble in ethanol, easily soluble in ether, chloroform and Acetic acid only dissolves 0.00072g in 100ml of water.
20 Flash point (ºC): >110
Toxicological data
1. Skin or eye irritation: human, skin contact, standard Draize test, 75mg/3D, mild reaction 2. Acute toxicity: Rat oral LD50: >10mg/kg; Mouse intravenous LC50: 57mg/kg
3. Carcinogenicity: Mouse transplantation TCLo: 1000mg/kg
Ecological data
General Notes
Generally not hazardous to water
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 77.73
2. Molar volume (cm3/mol): 287.3
3. Isotonic specific volume (90.2K ): 690.5
4. Surface tension (dyne/cm): 33.3
5. Polarizability (10-24cm3): 30.81
Compute chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): 6.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 14
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 37.3
p>
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 178
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is a white solid at room temperature and has the properties of saturated fatty acid. Insoluble in water, soluble in ether, petroleum ether, chloroform and other organic solvents.
2. This product is low in toxicity and irritating. Mice were intravenously injected with LD5057mg/kg. Operators should wear protective equipment.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and smoke.
?
Storage method
1. Packed in cardboard boxes or woven bags lined with plastic bags. Store in a cool, dry and ventilated place. Be careful to keep away from sources of fire and oxidants
2. Packed in cardboard boxes or woven bags lined with plastic bags, with a net weight of 25kg or 50kg per box (or bag). Store in a cool, dry and ventilated place, keep away from sources of fire and oxidants.
Storage and transportation in accordance with general chemical regulations.
Synthesis method
1. It is widely found in nature and can be found in almost all oils in varying amounts. Palmitic acid component. In my country’s specialty tallow tree oil, the content of palmitic acid can be as high as60%Above, while the content of palm oil in palm tree fruits is about40%, but the content in vegetable oil is insufficient2%. Palmitic acid is obtained by hydrolyzing tallow oil or palm oil, fractionating, and pressing to separate unsaturated fatty acids, and then recrystallizes them. The general melting point of commercially available palmitic acid is57.5-62.5 span>?.
2.Use suet or palm oil through hydrolysis and acidification,Palmitic acid can be obtained by separating unsaturated fatty acids, and then pure palmitic acid can be obtained by recrystallization.
3.Put the mixed fatty acids of rice bran oil, coconut oil, palm kernel oil, etc. through vacuum Produced by fractional distillation.
4. Tobacco: OR, 44; FC, 9, 15, 18, 41, 43, 50; OR, 18, 26; BU, 9, 18, 26.
Purpose
1. Mainly used in the production of soaps, candles, lubricants, softeners and synthetic detergents raw materials for the agent.
2.For the preparation of palmitate. Used as a waterproofing agent.
extended-reading:https://www.bdmaee.net/niax-catalyst-a-1/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-B-16-amine-catalyst-B16–B16.pdfextended-reading:https://www.cyclohexylamine.net/pc-cat-nmm-addocat-101-tertiary-amine-catalyst-nmm/extended-reading:https://www.newtopchem.com/archives/45013extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/65.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/31-12.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/139-1.jpgextended-reading:https://www.bdmaee.net/polyurethane-catalyst-a33-cas280-57-9-foaming-catalyst/extended-reading:https://www.morpholine.org/delayed-catalyst-1028/extended-reading:https://www.newtopchem.com/archives/39769

