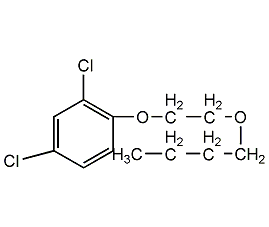
Structural formula
| Business number | 0286 |
|---|---|
| Molecular formula | C12H14Cl2O3 |
| Molecular weight | 277.14 |
| label |
2,4-D butyl ester, 2,4-butyl ester, 2,4-Dichlorophenoxyacetate butyl ester, 2,4-Dichlorophenoxyacetic acid n-butyl ester, 2,4-D butyl ester, Fernesta, Lironox, Butapon, herbicide |
Numbering system
CAS number:94-80-4
MDL number:MFCD00126845
EINECS number:202-364-8
RTECS number:AG8050000
BRN number:2056085
PubChem number:24869301
Physical property data
1. Properties: The pure product is a colorless oily liquid, and the crude oil is a brown liquid.
2. Density (g/mL, 20?): 1.2428
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 9
5. Boiling point (ºC, normal pressure): 146?147
6. Boiling point (ºC, 0.266KPa): 169
7. Refractive index: Undetermined
8. Flash point (ºC): >79
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC) : Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: Hardly soluble in water, easily soluble in most organic solvents.
Toxicological data
1. Acute toxicity: Rat oral LD50: 500?1000mg/kg
Mouse oral LD5O: 375mg/kg
Rabbit isLD5O: 1400mg/kg
Carp LC5O: 40mg/L(48h)
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment. Special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 67.63
2. Molar volume (cm3/mol): 226.2
3. Isotonic specific volume (90.2K) :554.2
4. Surface tension (dyne/cm): 36.0
5. Polarizability (10-24cm3): 26.81
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 35.5
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 236
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides and alkalis.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. In winter, antifreeze work should be done to prevent freezing. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. First, phenol is chlorinated to generate 2,4-dichlorophenol. After neutralization with an alkaline solution, it undergoes a condensation reaction with sodium chloroacetate to generate 2,4-D sodium salt. After acidification, it is esterified with butanol. The chemical reaction is obtained. Raw material consumption quota: phenol 480kg/t, chloroacetic acid 510kg/t, butanol 320kg/t, liquid chlorine 720kg/t, hydrochloric acid (30%) 520kg/t, liquid alkali (100%) 630kg/t.
2. The synthesis of 2,4-D is first to synthesize sodium phenolate and sodium chloroacetate, and then the two are condensed, and the reaction is at 110~120? Carry out, the reaction process keeps the solution slightly alkaline, and the feeding ratio of monochloroacetic acid and phenol is 1:1.16. Then a chlorination reaction is carried out, using chlorine gas as the chlorinating agent, and the reaction temperature is 92 to 98°C.
3. 2,4-Dibutyl ester Synthesis Add wet2,4- drops to a certain amount of butanol under stirring, raise the temperature to 120~140?, keep for 4h, fully dehydrate, and Alcohol refluxes. When the water level in the separator no longer rises, stop the reflux, raise the temperature to 160-170°C, and steam out the butanol under reduced pressure.
Purpose
1. Used as agricultural herbicide.
2.Broad-spectrum, hormonal herbicide with good spreading and systemic properties. Usually used in paddy fields and wheat fields, etc., mainly to control dicotyledonous weeds, heterosexual weeds and certain malignant weeds in grass fields, such as duckweed, eye cabbage, small three-edge grass, polygonum, wheatgrass, and ragweed. Grass, amaranth, pigweed, etc.
extended-reading:https://www.morpholine.org/pc-cat-tka-polyurethane-metal-carboxylate-catalyst-polycat-46/extended-reading:https://www.morpholine.org/morpholine/extended-reading:https://www.bdmaee.net/nt-cat-la-202-catalyst-cas31506-44-2-newtopchem/extended-reading:https://www.newtopchem.com/archives/44729extended-reading:https://www.bdmaee.net/toyocat-rx3-organic-amine-catalyst-tosoh/extended-reading:https://www.bdmaee.net/n-n-dimethylethanolamine-cas108-01-0-2-dimethylamineethanol/extended-reading:https://www.bdmaee.net/polyurethane-reaction-inhibitor-y2300-polyurethane-reaction-inhibitor-reaction-inhibitor-y2300/extended-reading:https://www.newtopchem.com/archives/category/products/page/165extended-reading:https://www.bdmaee.net/low-odor-reaction-type-9727/extended-reading:https://www.newtopchem.com/archives/44457

