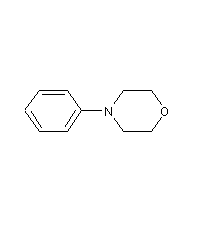
Structural formula
| Business number | 024U |
|---|---|
| Molecular formula | C10H13NO |
| Molecular weight | 163.22 |
| label |
N-phenylmorpholine, 4-Phenylmorpholine, (4-Morpholinyl)benzene, N-Phenylmorpholine, anti-corrosion additives, catalyst, Multifunctional solvent |
Numbering system
CAS number:92-53-5
MDL number:MFCD00006166
EINECS number:202-164-0
RTECS number:QE8575000
BRN number:None
PubChem number:24852668
Physical property data
1. Properties: colorless crystals.
2. Density (g/mL, 57/20?): 1.06
3. Relative vapor density (g/mL, air=1): 5.63
4. Melting point (ºC): 57
5. Boiling point (ºC, normal pressure): 268
6. Boiling point (ºC, 5.999kPa): 165~170
p>
7. Flash point (ºC, open): 104
8. Vapor pressure (kPa, 20ºC): <0.013
9. Solubility: soluble in ethanol , diethyl ether, insoluble in water.
Toxicological data
The oral LD50 in rats is 930mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 48.06
2. Molar volume (cm3/mol): 152.7
3. Isotonic specific volume (90.2K ): 381.0
4. Surface tension (dyne/cm): 38.7
5. Polarizability (10-24cm3): 19.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 12.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
N-Phenylmorpholine is a fairly stable compound and does not easily catch fire. Chemical properties pKa 3.20 (25°C, water). Condensation with benzaldehyde produces a colorless matrix, which is rearranged to obtain malachite green dye. Due to the presence of morpholino group, the ortho-para position of the benzene ring is activated and reacts with nitric acidObtain ortho and para nitro compounds. It is relatively stable to oxidation and only generates a small amount of formaldehyde when oxidized with dichromic acid.
Storage method
This product should be kept sealed.
Synthesis method
Prepared from the action of N, N-bis-?-hydroxyethylaniline and sulfuric acid.
Purification method: Use water for recrystallization and purification.
Purpose
The compound has properties similar to N, N-dimethylaniline and is basic. It can be used as a reagent for dehydrobromination of allyl bromide compounds and has the advantage that the product is easier to purify. Used as anti-corrosion additives, intermediates for dyes and insecticides, and catalysts in the decomposition of peroxides.
extended-reading:https://www.bdmaee.net/dimethylbenzylamine-cas-103-83-3-n-dimthylbenzylamine/extended-reading:https://www.newtopchem.com/archives/44053extended-reading:https://www.bdmaee.net/toyocat-rx3-organic-amine-catalyst-tosoh/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/9.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/NNN-trimethyl-N-hydroxyethyl-bisaminoethyl-ether-CAS-83016-70-0-Jeffcat-ZF-10.pdfextended-reading:https://www.newtopchem.com/archives/category/products/page/154extended-reading:https://www.cyclohexylamine.net/dabco-nem-niax-nem-jeffcat-nem/extended-reading:https://www.cyclohexylamine.net/category/product/page/28/extended-reading:https://www.newtopchem.com/archives/44674extended-reading:https://www.bdmaee.net/niax-ef-705-foaming-catalyst-momentive/

