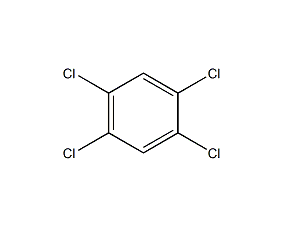
Structural formula
| Business number | 02A8 |
|---|---|
| Molecular formula | C6H3Cl3O |
| Molecular weight | 197.45 |
| label |
2,4,5-Trichlorophenol, 2,4,5-Trichlorophenol, 2,4,5-Trichloro-1-hydroxy-benzene, Aromatic halogen derivatives |
Numbering system
CAS number:95-95-4
MDL number:MFCD00002170
EINECS number:202-467-8
RTECS number:SN1400000
BRN number:607569
PubChem number:24889473
Physical property data
1. Properties: colorless needle-like crystals or gray flakes with a strong phenol smell.
2. Density (g/mL, 25?): 1.678
3. Relative vapor density (g/mL, air=1): 7.4
4. Melting point (ºC): 68
5. Boiling point (ºC, normal pressure): 246
6. Boiling point (ºC, 18mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, 72ºC) : 0.133
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure ( KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in carbon tetrachloride, alcohol, benzene, and ether.
Toxicological data
1. Acute toxicity: Rat oral LD50: 820mg/kg; Rat intraperitoneal LD50: 355mg/kg; Rat subcutaneous LD50: 2260mg/kg;
Mouse oral LD50 : 600mg/kg; mouse intravenous LD50: 56mg/kg; guinea pig oral LD50: 1mg/kg; mammal LD50: 150mg/kg;
2. Other multiple dose toxicity: rat oral TDLo: 98mg/kg/98D-C;
3. Chronic toxicity/carcinogenicity
Mouse skin contact TDLo: 6700mg/kg/16W-I;
4. Reproductive toxicity
Oral TDLo in mice: 4mg/kg (8-12 days after conception in female mice);
5. Mutagenicity
Microbiology Salmonella typhimurium mutation: 10?g/plate;
Cytogenetic analysis of hamster ovary: 150mg/L;
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment.
Molecular structure data
1. Molar refractive index: 42.81
2. Molar volume (cm3/mol): 123.7
3. Isotonic specific volume (90.2K): 329.8
4. Surface tension (dyne/cm): 50.5
5. Polarizability (10 -24cm3): 16.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 120
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants, acid anhydrides, and acid chlorides.
2. It is highly toxic and highly irritating if swallowed or inhaled. Can be poisoned by absorption through the skin. The oral dose for rats is LD50820mg/kg, and the oral dose for guinea pigs is LD501000mg/kg.
?
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from oxidants, acid anhydrides and acid chlorides, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. The wooden box outside the glass bottle is lined with padding or an iron drum. Store in a cool, ventilated warehouse; load and unload with care when handling to prevent damage to the container.
Synthesis method
Put 2,3,5,6-tetrachlorobenzene, solid alkali, and methanol into the autoclave, control the temperature at 135-152°C, and the pressure at 0.5-1.4MPa, and keep it for 14 hours. The reaction solution is cooled to 60°C, methanol is recovered by distillation, the residual liquid is released, cooled for crystallization, and filtered. Dissolve the crystals in water, heat to 70°C, add insurance powder, adjust pH = 9.2-9.6, add activated carbon, and decolorize at 95°C for half an hour. Filter, cool the filtrate to below 15°C, add hydrochloric acid to pH=2-3, filter out 2,4,5-trichlorophenol, and dry at 45°C to obtain the finished product.
Purpose
Used as fungicide and gas chromatography comparison sample.
extended-reading:https://www.newtopchem.com/archives/1031extended-reading:https://www.bdmaee.net/fascat9102-tertiary-amine-catalyst-triisocrylate-butyl-tin-arkema-pmc/extended-reading:https://www.newtopchem.com/archives/category/products/page/157extended-reading:https://www.newtopchem.com/archives/44701extended-reading:https://www.newtopchem.com/archives/44834extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/stannous-octoate-CAS-301-10-0–T-9.pdfextended-reading:https://www.morpholine.org/jeffcat-zf-10/extended-reading:https://www.morpholine.org/category/morpholine/page/6/extended-reading:https://www.bdmaee.net/drier-butyl-tin-oxide-fascat-4101/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/134-2.jpg