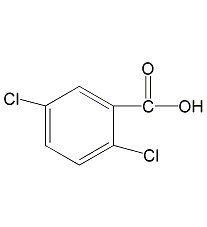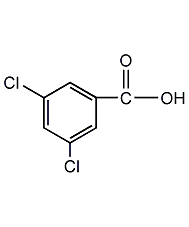
Structural formula
| Business number | 014Q |
|---|---|
| Molecular formula | C7H4Cl2O2 |
| Molecular weight | 191.01 |
| label |
3,5-Dichlorobenzoic acid, Benzoic acid, 3,5-dichloro- |
Numbering system
CAS number:51-36-5
MDL number:MFCD00002494
EINECS number:200-092-4
RTECS number:DG7350000
BRN number:2044776
PubChem number:24861653
Physical property data
1. Properties: brown powder.
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 188
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
1. Acute toxicity: mouse subcutaneous LD50: 250mg/kg; mouse abdominal LD50: 237mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 42.97
2. Molar volume (cm3/mol): 125.8
3. Isotonic specific volume (90.2K ): 341.1
4. Surface tension (dyne/cm): 53.9
5. Polarizability (10-24cm3): 17.03
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 10
6. Topological molecular polar surface area (TPSA): 101
p>
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 786
10. Number of isotope atoms ?0
11. Determine the atomic stereocenter??Number: 7
12. Number of uncertain atomic stereocenters: 0
13. Number of determined chemical bond stereocenters: 0
14. No Determine the number of stereocenters of chemical bonds: 0
15, the number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
None
Purpose
None
extended-reading:https://www.newtopchem.com/archives/1811extended-reading:https://www.bdmaee.net/nt-cat-nem-catalyst-cas100-74-3-newtopchem/extended-reading:https://www.newtopchem.com/archives/44417extended-reading:https://www.newtopchem.com/archives/1787extended-reading:https://www.newtopchem.com/archives/44451extended-reading:https://www.cyclohexylamine.net/dabco-xd-103-dabco-tertiary-amine-catalyst/extended-reading:https://www.bdmaee.net/cas%ef%bc%9a-2969-81-5/extended-reading:https://www.bdmaee.net/cas-616-47-7/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/3-7.jpgextended-reading:https://www.bdmaee.net/pc5-catalyst/