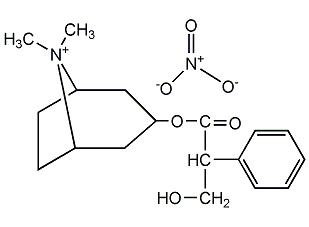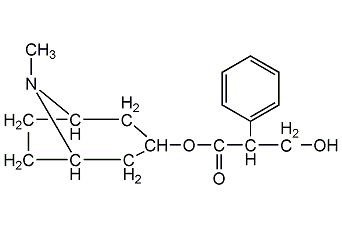
Structural formula
| Physical competition number |
0150 |
| Molecular formula |
C9H23NO3 |
| Molecular weight |
289.37 |
| label |
(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenyl-propionate,
belladonnaine,
Solanine,
m-methoxybenzaldehyde,
3-Methoxybenzaldehyde,
Solanin,
solanine,
Eggplant spirit,
Hyoscyamine,
Tropine tropate,
endo-(±)-?-(Hydroxymethyl)benzeneacetic acid 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester
|
Numbering system
CAS number:51-55-8
MDL number:MFCD00022622
EINECS number:200-104-8
RTECS number:CK0700000
BRN number:91260
PubChem number:24890401
Physical property data
1. Properties: White needle-like crystals. Sensitive to light and air. Sublime at 93-110? in high vacuum. 2. Density (g/mL, 25/4?): Not determined
3. Relative vapor density (g/mL, air=1): Not determined
4. Melting point (ºC): 2855. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point ( ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/ Log value of the distribution coefficient (water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: 1g is soluble in 455ml water, 90ml 80? water, 2ml ethanol, 1.2ml 60? ethanol, 27ml glycerol, 25ml ether and 1ml chloroform, also soluble in benzene and dilute acid. The pH of a 0.0015mol/L solution is 10.0
Toxicological data
1. Acute toxicity: Rat oral LD50: 500mg/kg
Mouse oral LD50: 75mg/kg
Ecological data
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. MooreEmissivity: 80.78
2. Molar volume (cm3/mol): 242.4
3. Isotonic specific volume (90.2K): 646.1
4. Surface tension (dyne/cm): 50.4
5. Polarizability (10-24cm3): 32.02
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 5
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 49.8
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 353
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 2
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure
Avoid light, open flame, and high temperature
Storage method
Filled with argon, sealed at 0 degrees Celsius, and stored in a dry place away from light.
Synthesis method
Hyoscyamine (L-body) is extracted from belladonna leaves, and then refined through racemization and recrystallization. It can also be obtained artificially.
Purpose
Atropine is a parasympathetic nerve inhibitor that can be used as an eye dilator and laxative; it can relieve hay fever, cold nasal obstruction and intestinal spasm; it can be used to treat nocturia in children, and is sometimes used to relieve ureteral and bile duct spasm; and It can be used to treat poisoning caused by organophosphorus. As an anticholinergic drug, atropine’s various pharmacological effects are not conducive to its clinical application. Some substitutes with specific effects have been synthesized, such as homatropine as a pupil dilator. Atropine is highly toxic and can cause blurred vision, stasis of secretion, vasodilation, high fever, excitement, agitation and delirium when the dose is too high. It is an antagonist of morphine, pilocarpine, physostigmine, etc.
Biochemical research. A reagent for assaying gold.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Addocat-108.pdf
extended-reading:https://www.newtopchem.com/archives/1006
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT2004-catalyst-CAS7772-99-8-stannous-chloride.pdf
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyltin-diacetate-CAS1067-33-0-dibutyl-tin-diacetate.pdf
extended-reading:https://www.bdmaee.net/amine-catalyst-smp/
extended-reading:https://www.newtopchem.com/archives/43936
extended-reading:https://www.bdmaee.net/trichlorobutyltin/
extended-reading:https://www.newtopchem.com/archives/45007
extended-reading:https://www.newtopchem.com/archives/44070
extended-reading:https://www.bdmaee.net/fascat-4200/