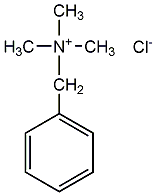
Structural formula
| Business number | 00JU |
|---|---|
| Molecular formula | C10H16ClN |
| Molecular weight | 185.70 |
| label |
N,N,N-trimethylphenylmethylammonium chloride, Trimethylbenzyl ammonium chloride, emulsifier, cellulose solvent, Polymerization inhibitor |
Numbering system
CAS number:56-93-9
MDL number:MFCD00011782
EINECS number:200-300-3
RTECS number:BO8400000
BRN number:3917255
PubChem number:24848426
Physical property data
1. Properties: White or light yellow crystals, easy to absorb moisture.
2. Density (g/mL, 20/4?): 1.072
3. Melting point (ºC): 239 (decomposition)
4. Refraction Rate (20ºC): 1.470
5. Solubility: Easily soluble in water, ethanol, hot benzene and butanol, slightly soluble in dibutyl phthalate and tributyl phosphate, insoluble in ether. Irritating.
Toxicological data
1. Acute toxicity: Rat oral LDLo: 250mg/kg; mouse oral LDLo: 1600mg/kg
2. Irritating to eyes, respiratory system and skin.
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 107
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Hygroscopic. It is stable below 135? and decomposes into benzyl chloride and trimethylamine above 135?. Protective clothing should be worn when using.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
The mixture of benzyl chloride, trimethylamine and methanol was refluxed for 4 hours, the methanol was evaporated to obtain a crude product, and the finished product benzyltrimethylammonium chloride was obtained through ethanol recrystallization with a yield of 88%.
Purpose
Reagents, emulsifiers, cellulose solvents, and polymerization inhibitors for the determination of platinum, palladium, mercury, and gold.
extended-reading:https://www.newtopchem.com/archives/category/products/page/122extended-reading:https://www.morpholine.org/category/morpholine/page/5404/extended-reading:https://www.newtopchem.com/archives/1002extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/67.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/31extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT2001-catalyst-CAS301-10-0-Stannous-octoate.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/38-2.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/145extended-reading:https://www.newtopchem.com/archives/748extended-reading:https://www.newtopchem.com/archives/1853

