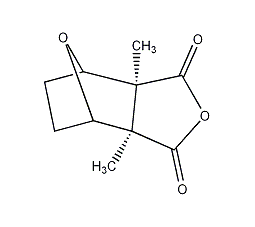
Structural formula
| Business number | 017D |
|---|---|
| Molecular formula | C10H12O4 |
| Molecular weight | 196.4 |
| label |
Hexahydro-3?,7?-dimethyl-4,7-oxyisobenzofuran-1,3-dione, Cantharidin, Hexahydro-3?,7?-dimethyl-4,7-epoxyisobenzofuran-1,3-dione, Inhibits protein and nucleic acid synthesis in cancer cells |
Numbering system
CAS number:56-25-7
MDL number:MFCD00134968
EINECS number:200-263-3
RTECS number:RN8575000
BRN number:None
PubChem number:24278334
Physical property data
1. Properties: This product is white crystal
2. Density (g/mL, 25/4?): Undetermined
3. Relative vapor density (g/ mL, air = 1): Undetermined
4. Melting point (ºC): 216?218?
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC ): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined Determined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in cold water, soluble in hot water, acetone, chloroform, ethyl acetate and oils, slightly soluble in ethanol.
Toxicological data
Ecological data
None
Molecular structure data
1. Molar refractive index: 45.68
2. Molar volume (cm3/mol): 144.0
3. Isotonic specific volume (90.2K ): 381.5
4. Surface tension (dyne/cm): 49.2
5. Polarizability (10-24cm3)?18.11
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds?: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 52.6
7. Number of heavy atoms : 14
8. Surface charge: 0
9. Complexity: 318
10. Number of isotope atoms: 0
11 , Determine the number of atomic stereocenters: 4
12. Determine the number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Sealed packaging in brown glass bottles. Store in cool and dark place.
Synthesis method
1. Cantharidin is a monoterpene substance extracted from the coleopteran insect Cantharidin of the genus Cantharidin. The content in adult insects is about 1%.
First, crush the cantharis into powder. Feed according to the ratio of cantharis coarse powder:concentrated hydrochloric acid:acetone=1:0.025:5.5. Take the dry coarse powder and add concentrated hydrochloric acid, then add 3 times the amount of acetone, soak and extract for 48 hours, then filter with double-layer gauze, collect the filtrate, and soak the residue in 2.5 times the amount of acetone for 48 hours, filter, collect the extracts, and combine the extractions liquid and discard the residue. The acetone in the extract is recovered to obtain a dark concentrated oil. A large number of crystals precipitate out of the oil. Filter and collect the crystals. The crystals are washed twice with petroleum ether to remove pigments and animal fats, then washed several times with 95% ethanol, and dried to obtain crude cantharidin. The crude product is recrystallized once with acetone to obtain cantharidin. Cantharidin is highly toxic, so precautions should be taken when preparing it. Green tea and coptis can detoxify it.
2. This product is a tincture made by drying the insect Cantharidin [Epicauta gorhami marseul (Meloidae)] and leaching it with ethanol. To prepare 1000ml of this product, first dry the cantharis, take 100g of the crushed coarse powder, add about 600ml of ethanol, stir occasionally to allow the soluble ingredients to fully dissolve, and filter with cloth. Then wash the residue with a small amount of ethanol and press dry. Combine the leachate and washing liquid, let it sit for 2 days and then filter. Add ethanol to the filtrate to make it 1000ml.
Purpose
1. Cantharidin can inhibit the protein and nucleic acid synthesis of cancer cells, thereby controlling their massive proliferation. It has certain efficacy in the treatment of primary liver cancer and is often used in conjunction with other anti-tumor drugs. In addition, it also has certain effects on breast cancer, esophageal cancer, leukopenia, lung cancer, chronic hepatitis and other diseases.
2.This product is only used in hair growth products. It is prohibited in other cosmetics. The maximum allowable content is 1%. External use to treat malignant sores, stubborn ringworm, psoriasis, and alopecia areata.
extended-reading:https://www.newtopchem.com/archives/44989extended-reading:https://www.newtopchem.com/archives/42989extended-reading:https://www.newtopchem.com/archives/751extended-reading:https://www.morpholine.org/nn-dicyclohexylmethylamine/extended-reading:https://www.cyclohexylamine.net/niax-nmm-jeffcat-nmm-lupragen-n105/extended-reading:https://www.newtopchem.com/archives/44027extended-reading:https://www.cyclohexylamine.net/cas-108-01-0-nn-dimethyl-ethanolamine-dmea/extended-reading:https://www.newtopchem.com/archives/category/products/page/129extended-reading:https://www.newtopchem.com/archives/category/products/page/37extended-reading:https://www.bdmaee.net/dimethyltin-dichloride/

