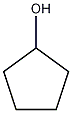
Structural formula
| Business number | 02B3 |
|---|---|
| Molecular formula | C5H10O |
| Molecular weight | 86.13 |
| label |
Hydroxycyclopentane, Hydroxycyclopentane, drug solvents, fragrance solvent |
Numbering system
CAS number:96-41-3
MDL number:MFCD00001363
EINECS number:202-504-8
RTECS number:None
BRN number:1900556
PubChem number:24857911
Physical property data
1. Properties: colorless viscous liquid with pleasant smell. [1]
2. Melting point (?): -19[2]
3. Boiling point (?): 140.4[3]
4. Relative density (water=1): 0.95 (20?)[4]
5. Relative vapor density (air = 1): 2.97[5]
6. Saturated vapor pressure (kPa): 252.2 (25?)[6]
7. Octanol/water partition coefficient: 0.71[7]
8. Flash point (?): 51 (CC)[8]
9. Solubility: Slightly soluble in water, soluble in ethanol, acetone, and ether. [9]
10. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3096.66
11. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -300.03
12. Liquid phase standard entropy (J·mol-1 ·K-1): 205.9
13. Liquid phase standard formation free energy (kJ·mol-1): -127.70
14. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3154.15
15. Gas phase standard claimed heat (enthalpy) ( kJ·mol-1): -242.55
16. Gas phase standard entropy (J·mol-1·K-1): 345.57
17. Gas phase standard formation free energy (kJ·mol-1): -111.45
18. Gas phase standard hot melt ( J·mol-1·K-1)?105.13
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity[10] IC50: 28~255mg/L (72h) (algae)
2. Biodegradability[11] Activated sludge method, 95% degradation in 5 days.
3. Non-biodegradability[12] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 1.5d (theoretical).
Molecular structure data
1. Molar refractive index: 24.64
2. Molar volume (cm3/mol): 85.7
3. Isotonic specific volume (90.2K ): 206.9
4. Surface tension (dyne/cm): 33.9
5. Polarizability: 9.77
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Interaction?Number of isomers: None
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 37.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain stereocenters of atoms: 0
13. The number of determined stereocenters of chemical bonds: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[13] Stable
2. Incompatible substances[14] Strong oxidizing agent
3. Polymerization hazard[15] No polymerization
Storage method
Storage Precautions[16] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37?. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency spill treatment equipment and suitable containment materials.
Synthesis method
1. Cyclopentanone is obtained by dry distillation of adipic acid under the action of sodium hydroxide. It is obtained by hydrogenating cyclopentanone and lithium aluminum tetrahydrogen in diethyl ether at room temperature. Or hydrogenate cyclopentanone in the presence of a chromium-copper catalyst at 150°C and 150 atmospheres or in the presence of a platinum catalyst at 0.2-0.3MPa to obtain a crude product, which can then be crudely distilled to obtain the finished product. Raw material consumption quota: adipic acid 2500kg/t, barium hydroxide 900kg/t.
2. Preparation method:
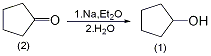
Into a reaction bottle equipped with a stirrer, thermometer, and reflux condenser, add 84g (1.0mol) of cyclopentanone (2), 750mL of diethyl ether and 150mL of water. Add 69g (3.0 mol) of metallic sodium (sodium wire and small pieces of sodium) as quickly as possible under vigorous stirring. You can cool the reaction bottle with ice water or rinse the reaction bottle with running water. When the metallic sodium has completely reacted, cool it, separate the upper ether layer, wash it with 25 mL dilute hydrochloric acid (1:1), then wash it with 25 mL water, dry it with anhydrous sodium sulfate or anhydrous potassium carbonate, fractionate, and collect 139~142 ? fraction, 78g of cyclopentanol (1) was obtained, with a yield of 90%. [18]
Purpose
Used as solvent and dye intermediate for spices and pharmaceuticals. [17]
extended-reading:https://www.bdmaee.net/butylstannic-acid/extended-reading:https://www.cyclohexylamine.net/catalyst-dabco-8154-acid-blocked-tertiary-amine-catalyst/extended-reading:https://www.cyclohexylamine.net/dabco-dc2-delayed-catalyst-dabco-dc2/extended-reading:https://www.cyclohexylamine.net/cas-33568-99-9-dioctyl-dimaleate-di-n-octyl-tin/extended-reading:https://www.newtopchem.com/archives/44885extended-reading:https://www.newtopchem.com/archives/44867extended-reading:https://www.bdmaee.net/dabco-nem-catalyst-cas100-74-3-evonik-germany/extended-reading:https://www.morpholine.org/high-efficiency-amine-catalyst-dabco-amine-catalyst/extended-reading:https://www.cyclohexylamine.net/spray-polyurethane-foam-catalyst-polycat-31-polyurethane-spray-catalyst-polycat-31/extended-reading:https://www.morpholine.org/polyurethane-catalyst-pc41/

