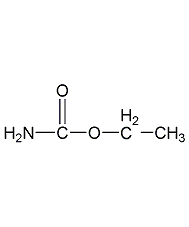
Structural formula
| Physical competition number | 0157 |
|---|---|
| Molecular formula | C3H7NO2 |
| Molecular weight | 89 |
| label |
urethane, Carbamic acid ethyl ester, Ethyl urethane |
Numbering system
CAS number:51-79-6
MDL number:MFCD00007966
EINECS number:200-123-1
RTECS number:FA8400000
BRN number:635810
PubChem number:24900632
Physical property data
1. Properties: Colorless and odorless crystals or white crystalline powder with a saltpeter-like smell.
2. Density (g/mL, 48/4?): 1.045
3. Relative vapor density (g/mL, air=1): 3.07
4. Melting point (ºC): 49
5. Boiling point (ºC, normal pressure): 182?184
6. Refractive index (52ºC): 1.4144
7. Refractive index (50ºC): 14.24
8. Refractive index (70ºC): 13.20
9. Flash point (ºC): 92
10. Heat of formation (KJ/mol): 1.664
11. Vapor pressure (kPa, 103ºC): 7.20
12. Vapor pressure (kPa, 120.7ºC): 14.40
13. Vapor pressure (kPa, 177ºC): 92.93
14. Solubility (%, 15.5ºC, water): 48
15. Solubility: soluble Soluble in water, ethanol, ether, chloroform and glycerin, slightly soluble in olive oil. It can dissolve almost all organic liquids except aliphatic hydrocarbons, such as aromatic hydrocarbons, esters, organic acids, alcohols and ethers.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 21.25
2. Molar volume (cm3/mol): 85.2
3. Isotonic specific volume (90.2K ): 205.2
4. Surface tension (dyne/cm): 33.6
5. Polarizability (10-24cm3): 8.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 52.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 52.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The aqueous solution is neutral. Potentially carcinogenic. It can be hydrolyzed by acid into ethanol, carbon dioxide and ammonium salt, and hydrolyzed by alkali into ethanol and cyanate. Can transesterify with higher carbon alcohols.
2.This product is toxic. The intravenous injection of LD50 into rabbits was 2000mg/kg body weight. The oral LD50 of white mice is 2700mg/kg body weight. Production equipment should be well-sealed to prevent escape, leakage, dripping, and leakage. Storage containers should be marked with toxic labels to prevent accidental ingestion. Operators wear rubber gloves. After skin contact, wash with water and soap; if ingested, seek medical attention promptly.
3. Exist in tobacco leaves and mainstream smoke.
4. LARC carcinogenicity assessment: sufficient evidence, triggering nuclear synergistic carcinogenic activity.
Storage method
Should be stored sealed in a cool, dry place.
This product is packed in cardboard barrels, lined with plastic bags, 25kg per barrel. Store sealed and away from light. Store in a cool, ventilated and dry place, and prevent fire, sun and moisture during transportation. Store and transport according to regulations on toxic chemicals.
Synthesis method
Nitric acid reacts with urea to generate urea nitrate, then ethanol and sodium nitrite are added, and in the presence of sulfuric acid, an esterification reaction is performed to generate urethane and sodium nitrate. The reaction product is filtered to remove sodium nitrate, and then distilled, crystallized, Drying to obtain the finished product.

Purpose
This product is used as an intermediate for medicines, pesticides, and spices, and for the production of sleeping pills and sedatives. It is used as an antidote for strychnine and resorcinol, a bactericide, a co-solvent for injections and a colorant for printing and dyeing industries. It can also be used in biochemical research. In addition, urethane itself can be used as a medicine, which has anti-cancer properties and is used to treat multiple myeloma and chronic leukemia.
extended-reading:https://www.morpholine.org/category/morpholine/page/2/extended-reading:https://www.morpholine.org/category/morpholine/page/10/extended-reading:https://www.bdmaee.net/pc-cat-td33-catalyst-triethylenediamine/extended-reading:https://www.newtopchem.com/archives/44405extended-reading:https://www.bdmaee.net/bisacetyloxydibutyl-stannan/extended-reading:https://www.bdmaee.net/cas-33568-99-9/extended-reading:https://www.newtopchem.com/archives/category/products/page/15extended-reading:https://www.bdmaee.net/polyurethane-catalyst-8154/extended-reading:https://www.cyclohexylamine.net/cas-1067-33-0-dibutyl-tin-diacetate/extended-reading:https://www.newtopchem.com/archives/44536

